BBI Contributing Editor
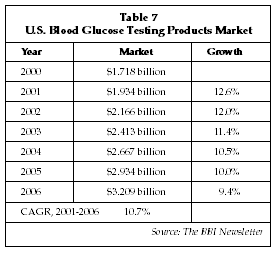
The market for whole blood glucose testing products used in the management of diabetes remains one of the highest-growth segments of the in vitro diagnostics market. The market is being driven by a number of factors, including increases in the worldwide prevalence of diabetes, a growing appreciation of the value of controlling blood glucose in improving outcome among both physicians and patients, and an in-flux of new technologies for glucose monitoring that facilitate more frequent and more widespread testing. As shown in Table 7, the U.S. market for whole blood glucose testing products, excluding the emerging market for implantable monitors, expanded by 12.6% in 2001 to over $1.9 billion. Worldwide, the 2001 market is estimated at approximately $3.9 billion, and suppliers expect the market to exceed $7 billion by 2010. Leading suppliers in the market include the Roche Diagnostics (Indianapolis, Indiana) unit of Hoffman-La Roche (Basel, Switzerland), the LifeScan (Milpitas, California) unit of Johnson & Johnson (New Brunswick, New Jersey), the Bayer Diagnostics unit of Bayer AG (Leverkusen, Germany) and Abbott Diagnostics (Abbott Park, Illinois).
 |
A major new development in the glucose self-testing market is the introduction of test systems featuring alternative-site sampling. Such systems are capable of determining capillary blood glucose levels using blood volumes of one microliter or less, allowing samples to be taken from the forearm, thigh or calf rather than from the fingertip, minimizing the pain of sampling. As a result, diabetics or, in the case of infant patients, their parents, are more receptive to testing. Since unit prices for the disposable microsampling sensor strips used for alternative site testing are equivalent to prices for conventional test strips, the overall dollar volume market is expanding. While there have recently been some issues raised regarding the accuracy of microsampling glucose test systems, in particular for measurements performed when glucose levels are changing rapidly, acceptance of the systems remains strong, and over the long term a significant proportion of the market is expected to convert to microsampling products.
Continuous or semi-continuous glucose monitoring systems also have recently been launched in the global market by companies such as MiniMed (Northridge, California), now a unit of Medtronic (Minneapolis, Minnesota) after more than two decades of research and development effort. Another supplier, Cygnus (Redwood City, California), has launched its GlucoWatch semi-continuous interstitial fluid glucose monitor outside the U.S. and recently obtained the final manufacturing approvals needed to launch the product in the U.S.
At present, continuous monitors can be used for only a limited period of time before the sensor must be changed and recalibrated. As a result, they are used to characterize trends in glucose level over the course of a few days, allowing the physician to recommend the optimal times for conventional sampling in order to optimize insulin dosing schedules, diet and lifestyle changes, and timing of fingerstick glucose measurements. Although sales of continuous/semi-continuous glucose monitoring systems remain very small at present, most industry experts expect sales of such systems to represent a more significant niche within the diabetes management products market in the future.
The arrival of new technologies has created upheaval in the market, since most of the companies developing new, less-invasive sampling technologies had not been leaders in the market previously. Acquisitions already have occurred that have allowed most of the major suppliers to access the growing microsampling segment. Other suppliers, such as Abbott, have now introduced their own microsampling products, leading to intense competition in this market segment. Further in the future, the possibility exists that completely noninvasive glucose monitors will be introduced, perhaps resulting in another wave of change in the market. Continued growth in demand appears inevitable, based on trends in disease incidence coupled with the fact that self-testing is considerably underutilized, particularly in developing regions of the world. According to Abbott Laboratories, only 1% of diabetics worldwide are testing their blood glucose with the frequency recommended by the National Institutes of Health (Bethesda, Maryland). And, according to the World Health Organization (Geneva, Switzerland), the number of people with diabetes will increase from the current level of 120 million to 140 million to more than double that figure by 2025, with previous estimates indicating that about three-fourths of all diabetics will live in developed countries.
Alternative sites gain ground
The first product to be launched for alternative-site glucose sampling was the AtLast system from Amira Medical (Scotts Valley, California). The AtLast system was cleared by the FDA in December 1998 and subsequently launched in retail outlets and other channels throughout the U.S. The AtLast system also offered an integrated approach to glucose testing, with the meter and sensor combined in a single unit. In November 2001, Amira was acquired by Roche Diagnostics, and in December 2001 Roche said that the AtLast system was being discontinued, to be replaced by the Accu-Chek Active, a new alternative-site sampling system developed by Roche. While convenient for users in that the lancet, test strip and meter were combined in an integrated unit, the AtLast system did not allow sampling from the fingertip in addition to alternative sites, an important factor in situations where glucose levels are changing rapidly. In addition, the new Active requires only half as much blood (one microliter vs. two microliters) and provides results in five seconds vs. 15 seconds with AtLast.
Another new entrant in the market, TheraSense (Alameda, California), introduced the FreeStyle glucose test system in June 2000. FreeStyle offers the smallest blood volume requirement of any glucose meter on the market, 0.3 microliters. Samples can be taken from the upper arm, forearm, thigh, calf and fleshy part of the hand, as well as from the fingertip. Nine of 10 people who used the FreeStyle in clinical trials found it to be less painful than their conventional fingerstick sampling system. TheraSense generated worldwide sales of $5.5 million in 2000, and sales increased to $79.1 million in 2001. The FreeStyle system is marketed in Europe by Disetronic Medical Systems (Burgdorf, Switzerland), the second-largest supplier of insulin infusion pumps. The product just received Ministry of Health and Welfare approval in Japan and Nipro (Tokyo) will market the product in that country. The rapid growth in sales for the FreeStyle system indicates the strong demand for glucose testing products that reduce the pain of sampling while still providing rapid, convenient results.
As a result of the burgeoning demand for microsampling glucose systems, most of the leading suppliers of glucose meters and test strips have developed their own product offerings in that area. In addition to the Roche Accu-Chek Active, both LifeScan and Abbott have recently introduced competitive microsampling products. Abbott has introduced the Sof-Tact system, which, like AtLast, integrates sampling and testing in a single device but uses a vacuum-assisted sampling technique that enhances blood perfusion in the skin. The Sof-Tact requires a 2-microliter to 3-microliter blood sample, and allows sampling from the top of the forearm and the outside of the upper arm. About 20 seconds are required to perform an analysis. Retail pricing for the Sof-Tact meter and test strips is essentially identical to that for Abbott's other glucose testing products.
LifeScan obtained FDA clearance for its OneTouch Ultra in August 2000. The Ultra was developed by Inverness Medical (Waltham, Massachusetts), a company acquired by Johnson & Johnson in late 2001 for $1.3 billion. While the Ultra does not integrate sampling and testing, it allows readings to be obtained with only one microliter of blood using electrochemical sensor technology, enabling tests to be performed using samples from the forearm, thigh, and other body sites. The measurement time is only five seconds. The Ultra was marketed by LifeScan prior to the acquisition of Inverness by J&J.
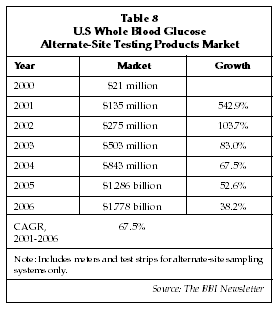
The new generation of microsampling glucose testing products is expected to capture a large and perhaps dominant share of the market worldwide. As evidenced by the reported revenue trend for TheraSense, a newcomer to the whole blood glucose market, sales of products allowing alternative site sampling are growing very rapidly. As shown in Table 8, sales of whole blood glucose alternative site sampling systems in the U.S. alone are expected to approach $1.9 billion by 2006, comprising more than half the sales in the U.S. glucose self-testing market. One possible negative factor in the microsampling products segment is recent concerns, raised by researchers as well as the FDA and suppliers themselves regarding inaccurate readings when glucose levels are changing rapidly in a patient. A study published by Theodor Koschinsky, MD, of the German Diabetes Research Institute at the University of Dusseldorf (Dusseldorf, Germany) in July 2001 presented data showing significant discrepancies between fingerstick glucose readings and readings taken from alternative sites such as the forearm using the TheraSense system in Type 1 diabetics whose glucose levels were intentionally manipulated to produce rapid changes. In some cases, the readings for alternative site samples were in the normal range, while readings from fingerstick samples were in the hypo or hyperglycemic range. Koschinsky concluded that a dangerous situation could potentially result wherein a diabetic on the verge of falling into a hypoglycemic coma would be given a false sense of security by an alternative site reading that indicated a normal glucose level.
 |
Manufacturers have since implemented changes in product labeling to warn users about potential discrepant readings. For example, TheraSense has added information to its labeling explaining that rapid changes in blood glucose levels will be detected sooner with fingerstick sampling than with sampling from other body sites. The company recommends that diabetics who are testing their glucose level because they think their level is low due to having symptoms of low blood glucose, or when a meal is delayed, or after taking insulin, or during or after exercise, use a fingerstick sample rather than one from an alternative site. Rubbing the alternative sampling site can also help to increase blood flow and allow the glucose level to equilibrate more quickly, although that technique is obviously subject to considerable variability, depending on how vigorously the user rubs the site. A study published by TheraSense demonstrated that the number of aberrant readings obtained in a typical diabetic patient is less than 1% of the total readings. However, the study also showed that it is possible to miss a rapid transition to a hypoglycemic state, leading to a recommendation to use fingerstick sampling if hypoglycemia is suspected.
The issue with lag time in glucose readings taken at alternative body sites is not likely to slow market growth for microsampling products significantly. Sales growth for suppliers such as TheraSense does not appear to have slowed. Type 2 diabetics who do not take insulin typically do not experience major, rapid transitions in blood glucose level and should encounter few problems with the lag effect, but are likely to benefit greatly from more frequent testing as a result of less painful sampling. Type 1 diabetics, although fewer in number than Type 2 diabetics, comprise a large segment of the market in terms of test volume and are also more likely to encounter situations where the lag time is an issue. However, they are also more likely to be able to recognize the symptoms of rapidly changing glucose levels and switch to fingerstick measurements when necessary. According to companies supplying products for diabetes management, there are also about 4 million Type 2 diabetics who use insulin to some extent and may possibly experience more rapid transitions in glucose level.
A niche for continuous glucose monitoring
Another recent development in diabetes management is the introduction of continuous glucose monitoring devices. The first device to be approved for marketing in the U.S. was the Medtronic MiniMed CGMS, which received premarket approval and was introduced in mid-1999. The CGMS uses a subcutaneous sensor that continuously tracks interstitial fluid glucose levels and stores the readings for subsequent download to a personal computer. The initial version of the CGMS is not available for consumer use, but instead is used by physicians to characterize a diabetic's daily pattern in glucose level over a three-day period, allowing the physician and patient to develop an optimal plan for insulin dosing, dietary intake, routine glucose testing, and lifestyle changes. MiniMed submitted an application for marketing clearance for a consumer version of the CGMS in August 2000, but that product remains under development. MiniMed is designing future versions of the CGMS as well as its external insulin pumps to allow for wireless data transfer, so that the pump and therefore the insulin dose could be automatically controlled by the sensor signals. MiniMed reported sales of $3.2 million for the CGMS product line in 2000, but sales in 2001 dropped to about a $2.4 million annualized rate based on reported results for the six-month period ended June 29, 2001.
Cygnus, the manufacturer of the GlucoWatch Biographer, is the second supplier that has succeeded in introducing a continuous glucose monitoring system. Cygnus launched the GlucoWatch outside the U.S. during 2001 and reported sales of $300,000 for the third quarter, all derived from the UK. Sales in the U.S. are planned to begin in the second quarter of this year, following an educational campaign directed at endocrinologists that is now under way. The product will be sold by Sankyo Pharma Inc., a U.S. subsidiary of Sankyo Co. Ltd. (Tokyo), the second-largest pharmaceutical company in Japan. Once the product becomes available in the U.S., it will be sold directly to consumers. The GlucoWatch uses a pad attached to the wrist that collects interstitial fluid drawn into the device via reverse iontophoresis, a process that is the reverse of the process used for iontophoretic drug delivery through the skin. While interstitial fluid glucose levels are not identical to capillary blood glucose levels, the GlucoWatch can be calibrated against a fingerstick glucose reading so that the glucose values displayed on the wrist-mounted monitor correspond to capillary glucose levels. The system is recalibrated every 12 hours with a capillary blood glucose reading. One important application for the GlucoWatch under study is detection of hypoglycemia. A study conducted at the Veterans Affairs Medical Center (San Diego, California) by Steve Edelman, MD, and others concluded that the GlucoWatch allows more effective detection of hyperglycemia than current medical practice. A major difference between the GlucoWatch and the Medtronic MiniMed CGMS is that glucose readings are displayed for the diabetic by the GlucoWatch, whereas the GCMS readings are stored internally and are later viewed by the physician.
As shown in Table 9, a number of other companies are developing continuous or semi-continuous glucose monitoring systems. Most are in preclinical development, although human studies have been conducted in some cases, including Technical Chemicals and Products (TCPI; Pompano Beach, Florida) and SpectRx (Norcross, Georgia). Disetronic is well-positioned among the companies targeting this market segment because of its strong position in the external insulin pump market. The TheraSense sensor technology being used by Disetronic also appears to have some advantages based on its small-volume testing capability, although configuring the sensor to provide the required biocompatibility represents a major new challenge. SpectRx also has demonstrated significant progress, working in collaboration with Abbott Laboratories, and in addition has received funding from the Centers for Disease Control and Prevention (Atlanta, Georgia). Roche also is involved in the development of continuous glucose monitoring systems, as well as the development of an automated pancreas that will link artificial intelligence, glucose monitoring, and insulin dosing. The market opportunity for continuous glucose monitoring systems is considerable, optimistically capturing a major share of the market for existing in vitro whole blood glucose testing systems, but the degree of penetration of that market will be very technology-dependent. Existing systems such as the CGMS or GlucoWatch do not eliminate the need for in vitro testing, and in fact depend on such measurements for calibration. Future systems such as the Disetronic/TheraSense device could prove particularly attractive to Type 1 diabetics, who comprise about 5% to 10% of all diabetics and are increasingly adopting external insulin pumps. More than 200,000 patients worldwide now use external pumps for insulin delivery, representing a market exceeding $170 million, excluding the cost of insulin. An automatic sensor-controlled pump could significantly improve convenience and quality of life for pump patients. It is likely that the market for that application alone could represent a $150 million opportunity worldwide. Clearly, continuous monitoring systems that eliminate the need for in vitro testing altogether and that are suitable for long-term use would address a much larger potential market.
 |
Noninvasive glucose testing
Noninvasive glucose measurement technologies have been under development for at least a decade by numerous companies. A variety of technologies have been studied, including various types of near-infrared spectroscopy, magnetic resonance, optical polarimetry and near infrared emission analysis. Companies involved in the development of noninvasive glucose measurement or diabetes management technologies include SpectRx, Roche, International Diagnostic Technology (Madison, Alabama), CME Telemetrix (Waterloo, Ontario) and InLight Solutions (formerly Rio Grande Medical Technologies; Albuquerque, New Mexico). SpectRx and Roche are jointly developing the Accu-Chek D-Tector, an optical device for noninvasive scanning of the eye to screen for the presence of diabetes. InLight Solutions has established a multi-year partnership with Johnson & Johnson to develop near-infrared glucose measurement technology and also is pursuing programs in the areas of blood alcohol testing, cancer detection and arterial blood gas analysis. CME Telemetrix is developing the GlucoNIR, a near-infrared glucose measurement system, funded in part by a 5% equity investment from Motorola (Northbrook, Illinois) in May 2000.
Although some companies, particularly InLight Solutions and CME Telemetrix, are continuing to progress toward the development of noninvasive glucose testing technologies, market introduction appears well in the future. The Accu-Chek D-Tector will be marketed by Roche following regulatory approval, but the product is a diabetes screening device, not a glucose monitor. It is likely that the emerging generation of microsampling glucose systems will come to dominate the market at least for the foreseeable future. The relative acceptance of the new products now on the market will be a key factor determining the future market share structure worldwide.
