CDU Contributing Editor
NEW ORLEANS – The management of heart disease has undergone a dramatic evolution over the past 20 years, driven by major developments in devices used for the treatment of vascular conditions and arrhythmia. As a result of the implementation of new device-based therapies, as well as advances in surgical and medical treatment of heart disease, deaths in the U.S. from diseases of the heart, as reported by the American Heart Association (AHA; Dallas), are now at approximately the same level as in the 1960s, in spite of a 55% increase in the U.S. population and an even greater increase in the population over age 65. Nevertheless, heart disease remains the number one cause of death in the U.S. Worldwide, ischemic heart disease ranked fifth as a cause of death in 1990, but that ranking is predicted to rise to No. 1 by 2020. Overall, an estimated 200 million people worldwide have clinically expressed cardiovascular disease, creating a large and growing demand for devices used in heart disease therapy.
A wide range of new developments in device-based therapy were described here at last month's 2004 AHA scientific sessions, ranging from new less-invasive technologies for the treatment of heart valve disease and advanced drug-eluting stents to new surgical approaches for heart failure therapy, as well as advances in device-based treatment for arrhythmia. New developments in diagnosis and risk assessment were also described at the AHA conference, particularly in the use of advanced diagnostic imaging modalities employing molecular targeting, which are expected to further expand the market for products used in heart disease management.
Minimally invasive heart valve repair
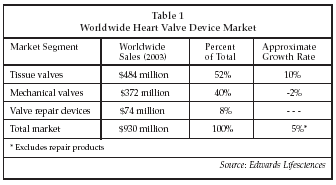
Device-based treatment for heart valve disease represents a significant segment of the total medical device market worldwide. As shown in Table 1 below, the worldwide market for prosthetic devices used in heart valve repair and replacement totaled $930 million in 2003, and is expanding at about 5% per year. The number of valve replacement procedures performed worldwide is expected to increase from 290,000 in 2003 to 850,000 by 2050. Leading suppliers in the prosthetic heart valve device market include Edwards Lifesciences (Irvine, California), Medtronic (Minneapolis), St. Jude Medical (St. Paul, Minnesota), Sorin Biomedica/Carbomedics (Milan, Italy), and ATS Medical (Minneapolis). A major new opportunity is about to emerge in the heart valve market for devices implanted via percutaneous techniques, as opposed to today's devices that require open surgery for implantation. Suppliers estimate the market for percutaneous therapies for valve disease could exceed $1 billion over the next decade. Percutaneous valve therapy is particularly advantageous for patients with aortic valve disease who are too sick to undergo open-heart surgery, or are unwilling to undergo invasive procedures. For patients with mitral valve disease, patients with moderate disease represent a large target population, since at present less than 10% of individuals in the U.S. with moderate to severe mitral valve disease have surgery. Key advantages of percutaneous valves over conventional surgically implanted valves include avoidance of sternotomy; use of off-pump, beating heart techniques rather than on-pump, stopped heart procedures; a procedure time of under two hours vs. about four hours for conventional valve surgery; and hospital stays of one to three days followed by a one week recovery period vs. stays of seven to 10 days and a recovery period of several months for conventional surgery.
Emerging percutaneous valve technologies described at the AHA sessions include a transcatheter annuloplasty device for mitral valve repair under development by Cardiac Dimensions (Kirkland, Washington). As discussed by Charanjit Rihal, MD, of the Mayo Clinic (Rochester, Minnesota) at a pre-AHA symposium organized by the Mayo Clinic, roughly one-third of the 5 million patients in the U.S. with congestive heart failure are symptomatic, and 17% of those or about 300,000 patients have severe mitral regurgitation. Most of those patients are high-risk surgical candidates, and consequently rarely undergo treatment now, but could potentially be treated with percutaneous techniques. Such treatment not only could improve quality of life, but may also improve clinical outcome, since recent studies cited by Rihal indicate that mitral regurgitation may exacerbate heart failure. The Cardiac Dimensions device uses proximal and distal nitinol anchors that capture the valve tissue and reshape the mitral valve to restore proper function. The device is currently undergoing studies in animals, with initial results demonstrating efficacy over a period of a few weeks, although some difficulties have been encountered in deployment of the device in convoluted coronary vessels, possibly leading to a need for pre-procedural selection of patients.
A second device for the treatment of mitral valve regurgitation in CHF patients is being developed by Viacor (Wilmington, Massachusetts). The company's Percutaneous Transvenous Mitral Annuloplasty (PTMA) device remodels the microannulus by placement of a stiff PTFE-coated nitinol/stainless steel device in the coronary sinus.
Percutaneous devices also are under development for the treatment of aortic and pulmonary valve disorders. Edwards Lifesciences, the leading supplier of conventional heart valves, has recently entered the race to develop a transcatheter heart valve via its acquisition of Percutaneous Valve Technologies (Fort Lee, New Jersey), a company founded by Alain Cribier, MD, of the University of Rouen. The device is being evaluated in the I-REVIVE study, and has produced a consistent improvement in hemodynamics in the patients receiving implants according to Helene Eitchaninoff of the University of Rouen, who discussed the technology at the AHA sessions. In its current configuration, the device is hand-crimped onto a balloon supplied by NuMed (Hopkinton, New York), and the heart is paced at 220 beats per minute during deployment to help stabilize the valve. Successful implants have been achieved in 12 of 14 patients to date in the I-REVIVE study, and additional implants have been performed by other investigators. At present, the device profile limits its use to patients having a vessel diameter of 7 mm or greater, but refinement of the design is expected to expand the range of patients who can be treated.
A number of other companies are involved in the development of percutaneous valve technologies. Devices include a nitinol valve with 4 micron thick leaflets fabricated using nanotechnology, described by Ruiz at a satellite symposium sponsored by Medtronic, and a second nitinol device from CoreValve (Paris). Mitralife/ev3 (Plymouth, Minnesota); Medtronic, with its Contegra valve; and Evalve (Redwood City, California) are other companies involved in percutaneous valve development.
As discussed at the AHA sessions by Magdi Yacoub of Middlesex Hospital in the UK, tissue-engineered valves represent another promising approach to improving heart valve repair and replacement therapy. Tissue engineering can potentially be used to fabricate devices that not only can be implanted via percutaneous techniques, but that can also grow with the patient, avoiding the need for re-operation in children who receive valve implants. Cook SIS (Bloomington, Indiana) is one of the companies developing a tissue-engineered heart valve, using its submucosal intestine material.
Another new approach to tissue-engineered valves was discussed by Ralf Sodian of the German Heart Institute (Berlin), which employs tissue engineering of autologous human heart valves. To fabricate the valves, vascular cells are isolated from harvested umbilical cords, cultured, and cryopreserved. The cells are then seeded onto a polymer scaffold supplied by Tepha (Cambridge, Massachusetts) that is formed into the proper shape using a computer-generated planning model and thermoplastic fabrication techniques. During the initial cell proliferation phase, the device is subjected to continuous pressure and flow cycles to stimulate growth of a structure that can withstand the hydrodynamic forces it will encounter once implanted. The resulting valve closely resembles a human heart valve, according to Sodian. If no pressure and flow cycling is employed, minimal cell growth is observed, indicating that mechanical stimulation is a key factor in directing development of a synthesized valve with the proper characteristics.
Membolix (also Cambridge) is yet another company involved in the development of tissue-engineered valves. As discussed by Doerthe Schmidt of University Hospital Zurich (Zurich, Switzerland) at the AHA sessions, a member of a research group led by Simon Hoerstrup, a series of increasingly complex tissue-engineered heart valves have been fabricated, first using cell seeding on a bioscaffold in sheep studies in 1999, and progressing to tri-leaflet valves in 2002. Most recently, the group has used endothelial progenitor cells derived from umbilical cord blood along with myofibrobasts from the umbilical cord to grow tissue-engineered valves on a polyglycolic acid scaffold. Pressure-flow cycling is used to direct cell growth. The resulting tissue construct is comprised of vascular cells and collagen, and control studies using cells not exposed to pressure-flow cycling results in a construct that lacks collagen, again demonstrating the importance of mechanical cycling in guiding proper development of the valve tissue.
Reperfusion, acute MI therapy developments
Another emerging emphasis in device-based therapy for heart disease is the development of technologies to enhance microvascular blood flow following a myocardial infarction, and to treat endothelial dysfunction that can predispose an individual to a heart attack even in the absence of a significant stenosis of the coronary arteries. The importance of endothelial dysfunction is underscored by statistics showing that greater than 60% of patients with an acute coronary syndrome have an angiographic stenosis of less than 50%. As discussed by Amir Lerman, MD, of the Mayo Clinic, at a Mayo-sponsored symposium held prior to the AHA sessions, cardiac stress testing is an important diagnostic method used to detect endothelial dysfunction in patients who have clinical symptoms of coronary artery disease, such as chest pain and heart failure, but do not show a significant stenosis on angiography. The recognition of the importance of microvascular and endothelial function has led to the development of new strategies aimed at restoring microvascular flow and myocardial perfusion after a primary blockage has been removed. A variety of therapies are being evaluated for their ability to eliminate atheroemboli and microvascular obstructions that cause additional myocardial tissue necrosis following a heart attack, in spite of restoration of primary blood flow.
Technologies under development include aqueous oxygen therapy, filters to capture thromboemboli released during flow restoration procedures, thrombectomy devices, tissue cooling, and a variety of thrombolytic drugs including agents to help prevent cellular apoptosis. Positive results were reported in the AMIHOT trial of aqueous oxygen therapy using a system being developed by TherOx (Irvine, California). The TherOx system consists of a cartridge that mixes a concentrated oxygen/saline solution with the patient's own blood, and a delivery catheter to infuse the mixture into the target vessels. While overall infarct size was not reduced in the AMIHOT study, there was a trend toward reduced infarct size in patients with anterior MI as well as an improvement in regional wall motion for patients who were treated with the TherOx system within less than six hours of symptom onset. In addition, some improvement in heart wall motion was observed in the entire cohort of treated patients. The results of clinical studies with other reperfusion techniques, such as tissue cooling, balloon occlusion, and various types of drugs, have been somewhat disappointing, in spite of initial positive results in animal trials. Multiple studies using various types of embolic capture devices have so far been negative, as were the results of the EMERALD trial that used the PercuSurge balloon protection system from Medtronic. In addition, a study using the AngioJet rheolytic thrombectomy catheter from Possis Medical (Minneapolis) to reduce infarct size in ST-segment elevation myocardial infarction STEMI patients treated with percutaneous intervention did not show an overall benefit for the device. While the results of the AiMI trial using the Possis device did not provide support for its routine use in STEMI patients, the company is planning further studies of patients with large thrombus burden, since the AiMI trial was not powered to detect a benefit of the treatment in that subset of patients. Trials of tissue cooling have employed a number of devices, including the Reprieve Endovascular Temperature Therapy System from Radiant Medical (Redwood City, California). While the results in the COOL MI trial using the Reprieve System failed to shown a decrease in overall infarct size, some benefit was observed in patients with anterior myocardial infarction whose temperature was reduced to below 35 C, leading the investigators to conclude that more rapid cooling and cooling to lower temperatures may be needed in order to derive significant benefit from tissue hypothermia.
Expanded indications for DES
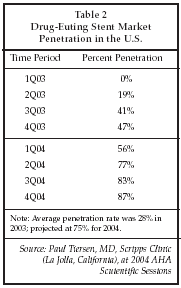
Drug-eluting stents (DES) continue to play a major role in expanding the utilization of device-based therapy for heart disease. As shown in Table 2, the use of drug-eluting coronary stents in the U.S. has risen from zero in 1Q03, prior to FDA approval of the Cypher stent from Cordis (Miami Lakes, Florida), to a projected 87% of coronary stent procedures in 4Q04. The penetration rate may not rise above 90%, at least with current-generation devices, since all patients who have implants of drug-eluting stents must undergo extended anti-platelet therapy to prevent thrombosis, and a certain percentage of patients cannot tolerate that therapy. Even that limitation may be removed, or at least minimized, in the future, as discussed by Paul Tierstein, MD, at the AHA sessions, if next-generation devices such as the Genous Bio-engineered R stent from Orbus Medical Technologies (Fort Lauderdale, Florida), are proven effective in reducing restenosis to the levels achieved by currently marketed drug-eluting stents. The Genous technology induces formation of a complete endothelial progenitor cell coating on the stent surface within 48 hours of implant, in principle providing a completely biocompatible covering that eliminates direct contact of the metal surface of the stent with blood, and thereby avoids activation of thrombogenesis. As a result, patients may not require long-term anti-platelet therapy, which would not only allow the remaining 10% of patients receiving coronary stent implants to achieve low levels of restenosis, but could also minimize the side effects and cost of anti-platelet therapy for other stent patients.
A number of additional next-generation devices are under development, according to Tierstein, including an amino acid-coated stent with a programmable biodegradation profile using polymer technology developed by Medivas (San Diego) and licensed by Guidant (Indianapolis) for use in drug-eluting stents. A Guidant stent using the Medivas polymer technology, which is designed to release nitric oxide into the vessel wall, has been evaluated in the NOBLESSE (Nitric Oxide through Bioabsorbable Layer Elective Study for Safety and Efficacy) trial with encouraging results. Such devices could further improve the safety and efficacy of drug-eluting stent therapy, and broaden the range of patients who can be successfully treated.
Yet another new approach to elimination of restenosis discussed at the AHA sessions by Fabao Gao of Johns Hopkins University (Baltimore) is the use of intravascular radio frequency (RF) heating plus vascular endothelial growth factor (VEGF) gene therapy to inhibit in-stent restenosis. VEGF treatment has been attempted by other researchers, according to Gao, but was ineffective because of low rates of gene transfection. Since heating of the target tissue has been shown to enhance transfection rates, Gao has employed a guidewire that is heated via radiofrequency energy to 41 C, and left in place for 20 minutes to heat the surrounding vessel tissue and thereby increase gene transfection. The VEGF gene therapy coupled with heat is delivered prior to stenting. In animal studies, neointimal hyperplasia was reduced to very low levels relative to controls when heat was applied. In addition, no reduction in restenosis was observed in the absence of VEGF infusion. The next step in the development program is to compare the VEGF/RF heat therapy to drug-eluting stents.
One topic under investigation for existing drug-eluting stents is the relative merits of the two devices now on the market, the Cordis Cypher stent and the Taxus stent from Boston Scientific (Natick, Massachusetts), in reducing restenosis, as well as the performance of the devices in various challenging patient subsets. The definitive head-to-head trial comparing the two stents, REALITY, involves 1,386 patients at 90 centers in Europe, Latin America and Asia. Although early data from the REALITY trial presented last March at the American College of Cardiology (ACC; Bethesda, Maryland) annual scientific sessions showed equivalence for deliverability of the two devices, complete results comparing efficacy in reduction of restenosis and adverse events such as thrombosis have not yet been released, and will probably not be presented until next March's ACC gathering, according to presenters at the AHA sessions. In the interim, initial data from two other ongoing trials show no statistically significant difference in revascularization rates between Cypher and Taxus, although there is a trend favoring Cypher. For example, 30-day data from a randomized comparison trial discussed at the AHA sessions by Miguel Romero of Reina Sofia Hospital (Cordoba, Spain), while based on a small subset of the 652 enrolled patients, shows a binary restenosis rate of 11% for Cypher vs. 16% for Taxus, and target lesion revascularization rates of 4% vs. 6%. Because of the small number of patients included in the initial analysis, the differences are not statistically significant. A second comparison trial, SIRTAX, was described by Stephan Windecker of Swiss Cardiovascular Center (Bern, Switzerland). SIRTAX has broad inclusion criteria, and has enrolled a total of 1,012 patients (503 with the Cypher and 509 with Taxus). At six months, the target lesion revascularization rates are 3.2% for Cypher vs. 4.7% for Taxus, and the target vessel revascularization rates are 3.4% vs. 5.5%. There have been three deaths in the Cypher group vs. 10 for the Taxus group at six months. Windecker said that the six-month data shows no statistically significant differences between the two stents in any category.
Thrombosis has surfaced as a potential drawback for drug-eluting stents due to higher-than-expected rates of thrombotic occlusion following market release of the Cypher stent. As discussed by Martin Leon, MD, of the Cardiovascular Research Foundation (New York) at the AHA sessions, subsequent experience in large-scale studies as well as registry data for the Cypher stent now show no difference in thrombosis rates for drug-eluting stents vs. bare-metal stents when recommended anti-platelet drug therapy protocols are followed. However, a small number of late thrombosis events (post-six months) were observed with both the Cypher and Taxus stents just prior to the AHA sessions, and thus late thrombosis remains a concern, particularly since it can have fatal consequences.
A number of studies were described at the AHA sessions that are assessing performance of drug-eluting stents in challenging patient subsets. For example, nine-month data from the TROPICAL (TReatment Of Patients with an Instent restenotic native Coronary Artery Lesion) trial, which is assessing performance of the Cypher stent in patients with in-stent restenosis and also comparing the results to those from prior vascular brachytherapy trials, were presented at the AHA sessions by Walter Desmet, MD, of University Hospital Gasthuisberg (Leuven, Belgium). According to Desmet, about 300,000 patients present with in-stent restenosis each year worldwide. Balloon angioplasty has proven relatively ineffective in treating in-stent restenosis, with a 20% to 80% recurrence rate for in-stent restenosis following angioplasty. Vascular brachytherapy was introduced in 2000, and initially appeared to offer a significant advance in dealing with the difficult problem of in-stent restenosis. However, subsequent experience has shown that the benefit of vascular brachytherapy diminishes over time. Drug-eluting stents now appear to be the most effective approach to treating in-stent restenosis, surpassing the efficacy of brachytherapy and minimizing (although not yet eliminating) issues with late thrombosis, as well as issues related to the logistics of delivering brachytherapy treatment.
In the TROPICAL trial, the six-month restenosis rate for Cypher was 9.7%, vs 40.3% restenosis in the GAMMA trials of vascular brachytherapy using gamma irradiation. The major adverse coronary event rate in TROPICAL at nine months was 6.2%, vs. 29.7% in GAMMA. Stent thrombosis was 0.6%, equivalent to the rate observed with bare metal stents in other studies. The nine-month target lesion revascularization rate was 4.9%, and late loss was less than 0.1 mm. A similar result was reported by Seung-Jung Park of the Asan Medical Center (Seoul, South Korea) in a comparison of drug-eluting stents and beta radiation brachytherapy for the prevention of in-stent restenosis. In a study of 129 patients, the restenosis rate for patients treated with the Cypher stent was 2.4% at six months, vs. a rate of 36.8% for the patients treated with beta brachytherapy. The results indicate that drug-eluting stents are the preferred treatment for in-stent restenosis, according to the investigators.
Data from a Cypher stent registry in Germany that includes more than 5,800 patients treated in 120 centers shows similar, if not better, results for drug-eluting stent treatment of in-stent restenosis as compared to those reported in brachytherapy studies. The target vessel revascularization rate in the registry at one year was reported at the AHA sessions at about 9% for patients with in-stent restenosis, the same as the target lesion revascularization (TVR) rate for patients with de novo lesions. However, the revascularization rates observed in the German Cypher registry are higher than those reported in the initial pivotal clinical trials of the Cypher stent, due to the inclusion of patients with more challenging lesions in the registry. Based on data from Germany, which has a total of 376 sites performing 210,000 percutaneous coronary interventional procedures annually, the penetration rate for drug-eluting stents has reached 78% in that country.
Another challenging patient subset for drug-eluting stents is patients with chronic total occlusions (CTOs). Results of treatment with bare-metal stents have been far from optimal, but drug-eluting stents may provide a solution for CTO patients. As discussed by Antonio Colombo, MD, of Centro Cuore Columbus (Milan, Italy) at the AHA sessions, the observed TLR in a series of CTO patients treated at his institution was 7.4% for drug-eluting stent therapy vs. 23.4% for bare-metal stents, with no stent thrombosis observed. The binary restenosis rates were 9.9% vs. 33.5%. The results demonstrate overwhelming better results for drug-eluting stents, according to Colombo, clearly making them the therapy of choice for patients with chronic total occlusions.
Perhaps the only significant barrier to drug-eluting stent adoption is the significantly higher cost of the devices as compared to bare metal stents. David Cohen, MD, director of interventional cardiovascular research at Beth Israel Deaconness Hospital (Boston) presented an analysis of the economics of drug-eluting stents, based on data from the Medicare program. The analysis includes all costs, including those due to additional procedures required as a result of higher restenosis rates for bare-metal stents. Cohen concluded that there is no added cost for drug-eluting stents if the incremental device cost vs. the cost of a bare-metal stent is $1,350. However, his data shows that the current average incremental cost is $1,800, indicating that hospitals are incurring about $450 in additional cost per patient for the use of a drug-eluting stent. If the improved quality of life associated with drug-eluting stent therapy is taken into account, however, including lower rates of angina, greater vitality and less post-operative pain in patients who avoid an additional revascularization procedure, the cost per repeat revascularization avoided is $10,000 at a bare-metal stent restenosis rate of 12%, which is well within the boundaries for cost-effectiveness of medical procedures in the U.S. Since actual bare-metal stent restenosis rates are 14%, the intervention is cost-effective, according to Cohen. Key cost-effectiveness issues for the future may revolve around the prophylactic use of drug-eluting stents in patients with multi-vessel disease, where the devices may prove useful in preventing an acute coronary event in lesions with vulnerable plaque.
Advances in heart failure therapy
Another major area of development focus for device suppliers in the heart disease treatment sector is heart failure therapy. A major new market has emerged for implantable electrical stimulation devices used in heart failure treatment. Implantable cardioverter defibrillator (ICD) devices have proven highly effective in slowing the progression of congestive heart failure, and in reducing the risk of death from the disease. For example, data presented at the AHA sessions on the Sudden Cardiac Death in Heart Failure (SCD-HeFT) trial shows a 23% reduction in mortality risk for patients undergoing ICD therapy vs. drug therapy. In spite of the high cost of ICD therapy ($17,500 for the device and lead according to data from a recent cost-effectiveness study), the efficacy of the treatment has resulted in rapid adoption, stimulating strong growth in the ICD market.
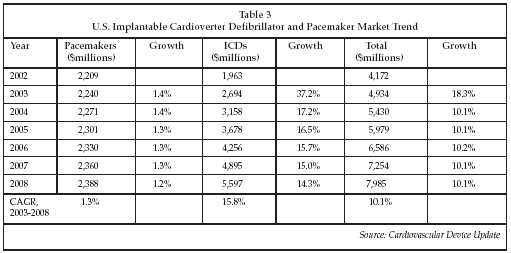
As shown in Table 3 below, the U.S. market for ICDs approached $2.7 billion in 2003, and is forecast to more than double to $5.6 billion by 2008. In contrast, the more mature market for cardiac pacemakers is forecast to grow from $2.24 billion in 2003 to slightly less than $2.4 billion by 2008. While costs for basic ICD therapy are roughly $24,000 including device and implantation procedure and hospital costs, the improvement in quality of life and reduction in hospitalization episodes makes the treatment cost-effective, a fact that was underscored by the recent issuance of a draft coverage decision by the Centers for Medicare & Medicaid Services (CMS; Baltimore) for ICD therapy for heart failure using the SCD-HeFT enrollment criteria as the coverage requirements, with the exception of the requirement for ejection fraction which was set at 30% rather than the 35% value specified in the trial. That decision was based in part on cost effectiveness data showing a $33,000 cost per life year added vs. a cost of $50,000 per life year for renal dialysis, a treatment already covered by the Medicare and Medicaid programs. Most ICD-CRT devices implanted today cost considerably more (about $42,000 for a full-feature device including implant costs), raising the issue of whether the Medicare reimbursement will cover the actual cost of treatment for many patients. Nevertheless, the decision is expected to result in continued strong growth in the ICD market.
ICD manufacturers are continuing to introduce advanced devices and accessories, improving ease of use and reliability of the devices, and helping to drive further market expansion. Guidant exhibited new bipolar ICD-CRT leads at the AHA conference, which provide the ability to select stimulation criteria that are configured for individual patients, and that allow electronic re-positioning and re-configuration of leads. Another advance from Guidant is the Latitude In Clinic program, which provides an electronic link between the ICD and an electronic medical record (EMR). Guidant has established a partnership with GE Healthcare (Waukesha, Wisconsin) for the EMR interface. The new program allows remote access by physicians to stored data on ICD activity. Future generations of the system will allow the patient to transmit ICD data to physicians via a modem or Internet connection, eliminating the requirement for the patient to visit a physician for data readout. Biotronik (Berlin) also exhibited a new ICD lead with a silicone coating that helps avoid tissue in-growth to facilitate removal, along with its automatic data uplink technology that allows ICD activity data to be wirelessly transmitted to physicians.
Acorn Cardiovascular (St. Paul, Minnesota) announced positive results from its trial of the CorCap device for heart failure treatment. The CorCap Cardiac Support Device is a mesh wrap that is surgically placed around an enlarged heart to help restore a more normal size and shape. The goal of treatment with the CorCap device is to minimize the abnormal stresses placed on the heart associated with dilated cardiomyopathy. Those stresses can themselves lead to further deterioration of heart function. Treatment with the CorCap device was shown to provide sustained improvement in heart size and shape, significant improvements in quality of life, and a 50% reduction in the likelihood that a patient would undergo additional procedures for progressive heart failure such as heart transplant and implants of ventricular assist or electrical stimulation devices.
Results of ongoing research aimed at biological restoration of heart function in CHF patients also were described at AHA. One of the most promising approaches uses cell-based therapy, and was discussed by Andre Terzic, PhD, of the Mayo Clinic. Key issues with cellular therapy for restoration of myocardial function have related to the ability of implanted cells to become integrated, both physically and electrically, in the heart, and to provide long-term, reliable augmentation of the heart's contractile capability. While some studies of cell implant therapy have produced improvements in heart function and reduction in symptom severity, most studies have not included randomized controls, raising the issue of placebo effects. In addition, researchers have generally implanted relatively small numbers of cells, in spite of the characteristically low survival of implanted cells, including autologous cells. That cautious approach is justified based on the outcome of some animal experiments that have produced large tumors, in some cases having a size equal to that of the host heart, when large numbers of cells were implanted.
However, limiting the number of cells implanted also limits the degree of improvement in cardiac function that can be achieved. Terzic has taken a step toward resolving such limitations by discovering a number of biochemical factors that are responsible for cell development, and using those factors during culture of cells destined for transplant to guide the cells down the cardiac myocyte development pathway. In addition, Terzic has succeeded in devising methods to control and arrest cell development, allowing the cells to be stored in an intermediate developmental state, such that they can be stimulated to differentiate into their final form once implanted. A patent application has been filed on the technology, and the researchers are assessing various options for commercialization, including tapping into venture capital sources to fund further development.
