CDU Contributing Editor
DALLAS – The 2005 scientific sessions of the American Heart Association (AHA), held here in the group’s home city in mid-November, provided a venue for presentation of the latest developments in heart disease management as well as a glimpse of emerging technologies that may play a role in improving the diagnosis and treatment of cardiovascular disease in the future. While no major breakthroughs were announced at this year’s conference, incremental advances in a variety of fields were described that collectively demonstrate the expanding range of technologies and products being employed in heart disease management.
Advances in diagnostic and monitoring technologies, as well as in imaging technologies, are helping to improve the ability to triage patients with cardiovascular disease and to guide therapeutic interventions more effectively. Emerging methods for the detection of vulnerable plaque or vascular risk, as well as new approaches for assessment of hypertension, were described at the conference that promise to improve the ability of physicians to determine the degree to which different patients will benefit from therapy, as well as to identify which therapies will be most beneficial for specific patients. The use of hypothermia in the treatment of patients with acute cardiovascular syndromes, while not a new concept, is gaining favor as advances in technology, coupled with an improved understanding of the therapy’s mechanism of action and optimal methods of use, have resulted in improved outcomes in recent studies.
Advances in interventional technologies, including minimally invasive approaches to heart valve repair and replacement as well as technologies for stroke prevention, were also highlighted at the AHA sessions. Recent progress in the use of stem cells and tissue engineering in the treatment of heart disease was discussed by a number of investigators at the AHA meeting, with some studies showing promise, although such technologies remain far from ready for widespread use in the clinic.
Blood pressure – no longer enough?
A new development in hypertension monitoring was announced at the AHA sessions in conjunction with presentation of the results of the Conduit Artery Function Evaluation (CAFÉ) study. The CAFÉ study was conducted as a sub-study of the landmark Anglo-Scandinavian Outcomes Trial (ASCOT) and assessed the effect of a new drug combination (amlidipine, a calcium channel blocker, plus peridopril, an ACE inhibitor) on patient outcome compared to patients treated with conventional beta blocker and diuretic drug therapy. The patients were monitored using both conventional cuff measurements of blood pressure as well as with a non-invasive monitor of central blood pressure, the SphygmoCor Px from AtCor Medical (West Ryde, Australia). The SphygmoCor device derives the central blood pressure waveform from a transcutaneous recording of the radial artery pressure waveform, and also computes a range of indices of arterial stiffness, cardiac function and ventricular-arterial interaction.
In the CAFÉ study, patients on the new drug regimen had significantly better outcomes than those on the conventional therapy, even though cuff pressures were the same in the two groups. Readings of central pressure from the SphygmoCor monitor, however, demonstrated a significant reduction in pressure of 4.3 mmHg for the group treated with the new drug combination compared to conventional therapy, providing a rationale for the improved cardiovascular outcomes that were observed. The researchers surmised that the difference in response to therapy might be due to a differing effect of the two drug treatments on vascular compliance. The findings indicate that more advanced measurement technologies than simple cuff readings of blood pressure have value in providing information that is more closely linked to cardiovascular risk. Similar results were obtained in the Strong Heart study, also presented at the AHA conference.
AtCor’s system is at present the only FDA-approved device for measuring blood pressure at the heart non-invasively. The company completed an initial public offering on the Australian Stock Exchange in early November, and reported a 25% increase in revenues for its fiscal year ended last June. AtCor has mainly focused on the use of its products for monitoring patients in clinical trials, but is now beginning a roll-out into the U.S. clinical practice market.
Another new device that goes beyond simple blood pressure measurement in providing non-invasive assessment of cardiovascular parameters was introduced at the AHA exhibition by Omron Healthcare (Kyoto, Japan). Omron’s HEM-9000AI system, which is now 510(k)-cleared, uses a multi-sensor array on the patient’s wrist to detect ejected pressure waves from the heart as well as reflected waves from the periphery. The readings, expressed as the Augmentation Index, can be linked to arterial stiffness and cardiac afterload, providing a more clinically informative picture of an individual’s cardiovascular status. Studies have demonstrated that the carotid Augmentation Index is an independent predictor of all-cause and cardiovascular mortality in end stage renal failure patients. The new Omron device, like the SphygmoCor, may alter clinical practice by enabling physicians to more accurately determine which treatments will provide the greatest benefit for patients.
A third technology introduced at the AHA meeting by Ostar Meditech (Riverside, California) takes non-invasive cuff measurements a step further in the ability to detect cardiovascular disease. The Ostar technology uses computerized sensors built into a blood pressure cuff to measure the energy spectrum of heartbeats. In normal individuals, the spectrum consists of a single peak, whereas multiple peaks appear if conditions such as arrhythmia, valve dysfunction, or coronary artery occlusions are present.
Three products based on the Ostar technology have been developed, including the P2, A2, and K7 Heart Condition Monitors. Development and initial clinical studies have been performed in Taiwan, and 510(k) clearance was obtained earlier in 2005. Ostar is in the process of establishing distribution in the U.S., and will market the device for use by physicians and by prescription to patients.
Another device available for non-invasive hemodynamic assessment that uses pressure pulse wave analysis is the DynaPulse system from PulseMetric (San Diego). In addition, Endothelix (Houston) is developing the Vendys system, which also uses a pressure cuff, but monitors temperature changes in the finger when blood flow is re-established after first being occluded by the cuff. The Endothelix device is not yet FDA-cleared but is being studied for assessment of vascular endothelial function, which may be linked to a wide range of diseases including stroke, dementia, sleep apnea, diabetes, heart attack, and renal failure, as well as hypertension and other conditions. Vasamed (Eden Prairie, Minnesota) is another supplier of non-invasive measurement devices for use in vascular disease assessment. Its SensiLase system is used to measure tissue perfusion levels with a laser-based analyzer and cuff. The device measures capillary flow, not arterial flow, by laser Doppler techniques, and is used in the assessment of patients with peripheral arterial disease.
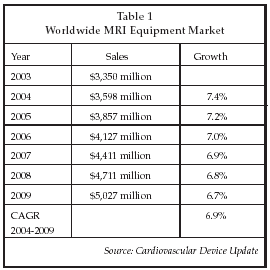
A number of advances in non-invasive imaging for cardiovascular disease diagnosis and therapy guidance were described at the AHA sessions. Magnetic resonance imaging (MRI) is perhaps the most rapidly evolving technology at present, with new applications described in areas as diverse as coronary restoration surgery, stem cell therapy, cardiac ablation for arrhythmia, vascular intervention and valve repair. Leading suppliers of MRI systems include GE Healthcare (Chalfont St. Giles, UK), Philips Medical Systems (Andover, Massachusetts), Siemens Medical Solutions (Erlangen, Germany), Toshiba Medical Systems (Tochigi, Japan), Hitachi Medical (Tokyo) and Fonar (Melville, New York). As shown in Table 1, the global market for MRI equipment is approaching $4 billion, and continuing to expand more rapidly than the overall market for medical imaging equipment.
A unique application of MRI that demonstrates the potential power of the technology was described by Wesley Gilson of Johns Hopkins University (Baltimore) at the AHA sessions. Gilson has demonstrated the ability to track stem cells transplanted into dog hearts by labeling the cells with iron particles prior to transplant. Iron labeling increases the sensitivity of detection of cells and also provides quantitation of the number of cells in the tissue.
There is considerable interest in methods to track stem cells implanted in the heart, particularly in non-invasive methods that could potentially be used routinely in patients, in order to develop an understanding of the process of integration of implanted cells into host tissue, and to monitor patients who undergo treatment. Gilson developed positive contrast and background suppression techniques that allow as few as 100,000 implanted cells to be detected. The technique also allows tracking of MRI-compatible endovascular devices such as stents.
Another study, discussed by Jiangyang Zhang of Johns Hopkins, used the Feridex MR contrast agent developed by Advanced Magnetics (Cambridge, Massachusetts) and marketed by Berlex Laboratories (Montville, New Jersey), which is based on superparamagnetic iron oxide. Zhang showed that bone marrow progenitor cells labeled with Feridex can be tracked when they migrate to the site of a vascular injury. The study was conducted in mice that were subjected to an induced injury in the femoral artery.
One symposium at the AHA meeting focused on the emerging field of interventional MRI, which involves the use of MRI to guide the use of devices such as catheters in performing interventional procedures. Such technology has shown promise in allowing interventions involving soft tissue to be more precisely directed, since the key characteristic of MR imaging is its ability to differentiate soft tissue. In addition, MRI eliminates radiation exposure issues associated with X-ray imaging for guidance of interventions.
Interventional MRI has evolved slowly, with one of the pioneers in the area, Surgi-Vision (Columbia, Maryland), a company formed by researchers from Johns Hopkins, no longer in business. However, some promising new developments were discussed at the AHA conference, including a collaboration involving GE Healthcare and Boston Scientific (Natick, Massachusetts) to develop interventional MRI for a variety of applications in interventional radiology.
In studies discussed by Michael McConnell of Stanford University (Palo Alto, California), MRI was used to guide penetration of a guidewire through a chronic total occlusion, viewing the wire in 3-D in essentially real time. According to McConnell, X-ray angiography is not adequate for optimal guidance of such procedures, or for applications such as TIPS (transjugular intrahepatic portosystemic shunt) procedures and characterization of arterial plaque, due to its lack of ability to image soft tissues. In the case of TIPS, for example, multiple target vessels are visible on the MR image that typically are not detected in the X-ray image.
In the studies discussed by McConnell, optically isolated surface coils are used to provide signal enhancement. A further advantage of MRI in such applications is that no contrast agent is needed, avoiding issues with toxicity that can arise with x-ray contrast agents.
A new imaging modality with potential applications in assessment of patients with cardiovascular disease was described by Douwe Mulder, MD, of University Medical Center (Groningen, the Netherlands). The technology employs a fluorescence analyzer developed by Diagnostics (also Groningen) that measures skin autofluorescence due to advanced glycation endproducts (AGEs). AGEs are believed to arise from oxidized lipid and glucose, and are a putative marker of oxidative stress. Studies have demonstrated a correlation between autofluorescence in skin biopsies and the presence of cardiovascular disease. In particular, skin autofluorescence is elevated in patients who have ST-elevation myocardial infraction, and gradually decreases as patients recover from the event.
Skin autofluorescence also is associated with elevated levels of established risk markers for myocardial infarction and diabetes such as C-reactive protein and hemoglobin A1c. Mulder and his co-researchers believe skin autofluorescence is a potential tool for assessing inflammation and/or oxidative stress in high-risk patients. The technique appears to be broadly applicable, although difficulties have arisen in studying patients with dark skin.
Detection of vulnerable plaque is another potential application for imaging technologies in heart disease management. Intravascular MRI catheters are one option under development, since MRI is known to be an effective modality for soft tissue characterization. Other technologies for vulnerable plaque detection under development include near-infrared imaging, optical coherence tomograhy, intravascular ultrasound, palpography, Raman spectroscopy, and CT angiography.
InfraReDx (Burlington, Massachusetts) is developing near-infrared technology for vulnerable plaque detection using a fiber-optic, catheter-based system. The company’s Thin Cap Fibrous Atheroma Detection (TCFAD) system consists of an optical analyzer and a single-use catheter designed to detect large lipid deposits on the walls of the coronary arteries that are presumed to be markers for vulnerable plaques.
Vital Images (Minnetonka, Minnesota), an established supplier of advanced visualization and analysis software for disease screening, clinical diagnosis, and therapy planning, is another company pursuing development of vulnerable plaque detection technology. The company is involved in a collaboration to develop new image analysis software that can be used with CT angiography imaging to detect vulnerable plaque. As discussed by Robert Schwartz of the Mayo Clinic (Rochester, Minnesota) at the AHA sessions, CT angiography, a non-invasive method of assessing the coronary arteries, preferentially detects calcified plaque, which is often believed to be stable, rather than vulnerable, because it is more firm and less prone to rupture. However, studies cited by Schwartz show that calcific plaque is not necessarily stable, and can consist of regions of calcium surrounding regions of lipid-rich plaque. A study of patients with chest pain showed that more than half (56%) had some regions of uncalcified plaque, and experienced chest pain even though there was no severe stenosis present in 51%. As a result, CT angiography may also prove to be a valuable, and more importantly a non-invasive, tool to search for vulnerable plaque.
Advances in minimally invasive heart valve repair
Percutaneous technologies for repair and replacement of heart valves are also poised to drive expansion of the cardiovascular device market. A number of companies are developing heart valve repair and replacement devices for percutaneous use, as shown in Table 2 below. Development is at an early stage, although the number of patients who have received implants in clinical trials now numbers in the hundreds. Subjects in those trials have generally been limited to those with no other options for valve repair, such as patients who are unable to undergo surgery, and who would have a limited life expectancy if left untreated.

Some percutaneous valve development programs have encountered difficulties due to the complexity of the implant procedure as well as issues with device retention. Clinical trials of Edwards Lifesciences’ (Irvine, California) Cribier-Edwards percutaneous aortic valve were halted for about six months to revise the device diameter and implant technique as a result of adverse events occurring with the original design, but the trial recently resumed. Use of advanced CT imaging to obtain accurate measurements of diameter prior to implant has helped improve procedural success rates in some trials.
In addition, newer approaches incorporate technologies designed to improve the guidance of the implant process, such as the magnetically guided device under development by Mitralign (Salem, New Hampshire). In addition to Edwards, other established suppliers of heart valve devices such as Medtronic (Minneapolis) and Shelhigh (Union, New Jersey) are also are developing minimally invasive heart valve repair and replacement technologies.
Minimally invasive surgical approaches to valve repair also are under investigation. For example, Myocor (Maple Grove, Minnesota) is developing the Coapsys Annuloplasty System for repair of mitral regurgitation employing minimally invasive surgery. As discussed by Rany Chitwood, MD, of East Carolina University (Greenville, North Carolina) at the AHA sessions, video-directed endoscopic surgery for valve repair also is experiencing growth. About 50 centers now are performing robot-assisted mitral valve surgery according to Chitwood, and at least five of those centers are performing all mitral valve surgeries using robotic guidance. At the Cleveland Clinic, almost two-thirds of the 3,163 mitral valve cases performed in a recent period were done by minimally invasive techniques, resulting in fewer blood transfusions, shorter length of stay, lower short-term mortality rates, and long-term outcomes that are equivalent to those achieved with open surgery. The advantages of minimally invasive techniques appear to be related to short-term outcome so far.
The number of companies involved in developing minimally invasive valve repair and replacement technology has expanded rapidly, making the field quite crowded even though a commercial market has yet to develop. However, suppliers such as Edwards have recently estimated the global market at $800 million in the long term for minimally invasive devices to treat aortic valve disease alone, and an additional market opportunity exists for treatment of pulmonary and tricuspid valve disease, so the market can potentially support a number of competitors.
Eventually, if developers achieve their objectives, the market may expand to include patients who are not treated surgically at present because their disease is at an early stage, and the risk of surgery outweighs the potential benefit of treatment. Minimally invasive technology can potentially be safer than open surgery, and allow such patients to become candidates for treatment.
Progress reported in tissue engineering
Another area of continuing research focus highlighted at the AHA conference is the use of tissue engineering for treatment of cardiovascular disease. A number of researchers described results using stem cell therapy for regeneration of damaged heart tissue in patients with congestive heart failure. In addition, tissue engineering is being employed to develop new types of vascular grafts that may lead to improved coronary artery and peripheral vascular bypass treatments and improved dialysis access.
Volker Sch chinger, MD, of J.W. Goethe University (Frankfurt, Germany), described the use of bone-marrow derived stem cells for treatment of heart failure in the Intracoronary Infusion of Bone Marrow-Derived Progenitor Cells in Acute Myocardial Infarction: A Randomized, Double-Blind, Placebo-Controlled Multicenter Trial (REPAIR-AMI). REPAIR-AMI, which has enrolled 204 patients at 17 medical centers in Europe, is the largest randomized, placebo-controlled trial of stem cell therapy for heart failure that has been conducted to date. The treatment involved isolation of autologous bone marrow cells and reinfusion of the cells into damaged tissues. A statistically significant increase in ejection fraction was observed at four-month follow-up in the treatment group, from 48% to 54%, vs. controls that received a placebo injection, the latter showing an increase from 47% to 50%. While the differences are small on an absolute basis, the treated patients also showed benefits in terms of reduced enlargement of the heart, and improved blood flow.
While the study was not powered to show a difference in death rates or revascularization, there was a reduction in the composite endpoint including death, new MI, and hospitalization due to heart failure. In addition, there was no increase in overall adverse events in the treatment group. Patients who had larger myocardial infarctions, as indicated by those with ejection fraction below 50%, showed more benefit from treatment. A new finding of the study related to the timing of cell transplantation. Patients who received cell therapy more than five days after the initial infarction showed greater benefit than those treated earlier.
Controversy continues to exist about the mechanisms that are responsible for improvements in outcome observed with stem cell therapy for heart failure. Also, some studies have not shown a statistically significant benefit, although because of the relatively small benefits observed, minor variations in methods could result in differing outcomes.
Improvements in stem cell therapy will depend in part on the results of ongoing research to elucidate the mechanisms involved in producing benefit, which will help determine the best strategies to pursue. For example, Michael Schneider, MD, of Baylor College of Medicine (Houston), described research under way at Kardia Therapeutics (also Houston) to develop directed-growth technologies to increase the number of cardiac cells produced by transplant therapy. The company, founded by doctors from Baylor, including Schneider, has identified cardiac progenitor cells that exhibit homing behavior to damaged cardiac tissue, as well as drug treatments that can be used to promote cell differentiation. The company is continuing to analyze the factors involved in cardiac muscle cell creation to allow development of more potent treatments. According to Kardia, there are more than 3 million hospitalizations annually related to heart failure, and combined expenditures for related inpatient and outpatient care total over $20 billion per year.
Another application of tissue engineering in cardiovascular therapy was described by Todd McAllister, PhD, of Cytograft Tissue Engineering (Novato, California). The company has developed biologically engineered blood vessels created from autologous cells that could potentially be used in coronary artery bypass graft surgery for patients who do not have blood vessels suitable for harvest. Cytograft had previously reported on animal experiments with grafts created using its sheet-based tissue engineering technology, which involves growth of a layer of cells around a cylindrical mandrel and isolation of the cell construct for use as a replacement blood vessel.
At the AHA sessions, McAllister described the first human studies with the Cytograft tissue-engineered blood vessels. The initial application under development is use of engineered blood vessels for dialysis access grafts. Autologous cells are derived from a skin biopsy taken from the back of the patient’s hand. The biopsy is processed to obtain a sheet of cells and extracellular matrix that is rolled onto a tubular support and cultured. A total of nine patients have received grafts so far in a trial being conducted in Argentina. The engineered vessels average 4.8 mm in diameter, and have mechanical properties that are intermediate between those of veins and arteries, exhibiting suture retention strength and burst pressure that are adequate for in vivo use.
At five-month follow-up, the first three patients treated have not experienced any graft failures, and the grafts are functioning well as dialysis access conduits. Importantly, sealing of the grafts after puncture for the dialysis procedure occurs more quickly and with greater reliability than with ePTFE grafts, which are widely used now for dialysis access. The grafts also appear to adapt to their environment after implant, showing increased compliance over time. However, some decrease in diameter (27% at five months) was observed, which might indicate that stenosis of the graft could be a possible long-term failure mode.
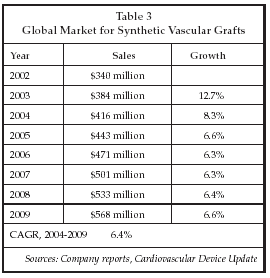
Another group, led by Simon Hoerstrup of University Hospital Zurich (Zurich, Switzerland), described experiments with tissue engineered pulmonary arteries implanted in sheep that for the first time have shown growth of the vessels following implantation. Implanted cells have been shown to express functional markers that are the same as those seen on native cells, and the tissue is actually stronger than native vessels. Hoerstrup’s technique employs a biodegradable polymer as a scaffold that is seeded in vitro, and that dissolves rapidly once the device is implanted, leaving only autologous cells. Follow-up in animal studies is more than 100 weeks. As shown in Table 3 below, tissue-engineered vascular grafts could address a market that is expected to approach $600 million worldwide by the end of the decade.
Hypothermia device market grows
The use of hypothermia to improve outcomes for patients who suffer a cardiac arrest was another topic addressed at the AHA sessions. Ken Nagao, MD, of Nihon University School of Medicine (Tokyo), discussed results of a preliminary study of patients who were prospectively cooled prior to responding to cardiopulmonary resuscitation (CPR). Cooling has already been shown to reduce neurologic damage if used after patients respond to CPR, but it was not known if cooling prior to response to CPR in patients who subsequently had a return of spontaneous circulation would have a benefit. The study showed that both groups of patients had similar neurologic outcomes, lending support to an expanded use of hypothermia treatment.
Hypothermia for stroke patients, for temperature reduction in patients with fever and for patients undergoing cardiac and neurosurgery is now an accepted treatment modality. The first products with such indications were cleared by the FDA in 2002, and a number of additional products have received clearance since then, the most recent being the ICY Catheter from Alsius (Irvine, California). Other FDA-cleared devices are available from InnerCool Therapies (San Diego), Radiant Medical (Redwood City, California) and Medivance (Louisville, Colorado). Typical pricing, as exemplified by the InnerCool catheter, is about $1,900 per procedure.
In spite of the growing evidence of benefit, however, a recent survey found that only 36% of non-physicians and 26% of physicians are using hypothermia for cardiac arrest patients. Reasons given for not using hypothermia include lack of data on its effectiveness, the difficulty of performing hypothermia, and the fact that use of hypothermia in cardiac arrest is not recommended in published guidelines.
Nevertheless, there is significant interest in using hypothermia in cardiac arrest patients in some circles. Advocates of the technique believe that, in order to achieve significant benefit, it is important to initiate cooling as early as possible and to ensure that a reduced temperature is accurately maintained. Some studies that have had negative results have employed cooling methods that have not been validated as providing an adequate and consistent temperature reduction.
Thus there appears to be an opportunity for suppliers of devices that can quickly induce a drop in body temperature and maintain reduced temperature accurately, with a temperature of 28° C being a target quoted by some suppliers.
As discussed by Francis Kim, MD, of Harborview Medical Center (Seattle), one approach that is relatively easy to implement is the use of cooled saline to induce hypothermia in cardiac arrest patients while they are still in the ambulance. Kim has performed a study of 100 patients in the Seattle area treated with hypothermia using cold saline infusion along with passive cooling approaches. So far, the results of the study have shown that hypothermia is safe. While no statistically significant difference in outcomes has been identified between patients receiving hypothermia and controls, a subgroup analysis of patients who suffered cardiac arrest due to ventricular fibrillation (VF) showed improved survival. That outcome supports the 2003 recommendations of the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation for use of hypothermia (at 32° C to 34° C) for 12 to 24 hours in unconscious patients with spontaneous recirculation after out-of-hospital cardiac arrest when the initial rhythm was VF. Improved outcomes may be achieved by cooling to a lower temperature than can typically be produced with IV ice slurry infusion, which results in a drop of about 2°C.
As a result of increasing interest in the use of hypothermia, the field is attracting some new entrants. A new device for inducing hypothermia was exhibited at the AHA meeting by Life Recovery Systems (Alexandria, Louisiana), a privately held and privately funded company with research facilities in Kinnelon, New Jersey. The company’s Thermosuit con00sists of a disposable plastic body suit that allows a thin layer of ice water to be circulated in direct contact with the patient’s skin. In experiments using swine models, the Thermosuit was able to lower core temperature to 29° C within 30 minutes, and produced a 4° C drop in eight minutes, compared to a 2° C drop for an IV ice slurry and 1.5° C for the Radiant catheter. The Thermosuit is now undergoing FDA review for use in hypothermia procedures.
