CDU Contributing Editor
ATLANTA, Georgia – The interventional cardiology products market, already one of the largest and fastest-growing segments of the medical device market worldwide, is becoming increasingly diverse as new product segments continue to emerge. The market for coronary stents is by far the largest segment and is expected to exceed $2.8 billion in the U.S. alone by 2006 as shown in Table 1. Drug-eluting stents are about to enter the market in Europe and probably will hit the U.S. market in mid-2003, providing a major growth stimulus. However, other new technologies promise to allow expansion of the market into new territory, further eroding the domain of cardiac surgery as well as that of the vascular surgeon. Not only is the combination of drugs and stents in a single device proving to be highly effective in vascular therapy, but in addition systemic drug therapy in combination with stent treatment is allowing outcomes to be achieved that are in some cases superior to those of surgical treatment. The latest advances in interventional cardiology, presented here at the 51st annual scientific sessions of the American College of Cardiology (ACC; Bethesda, Maryland), indicate that rapid progress is likely to continue in the future.
 |
While drug-eluting stents appear, at least for now, to be the solution to the problem of restenosis that has plagued interventional therapy since its inception, a large pool of patients who have first-generation stents exists. Those patients are expected to drive additional demand for continually evolving technologies for the treatment of in-stent restenosis, at least for the next few years. Along with the growth in procedure volume due to continued conversion of patients from surgery, at least 20% to 30% of patients who have already had stent implants are expected to return for treatment of in-stent restenosis. While drug-eluting stents may also provide the solution to treatment of in-stent restenosis, existing technologies, most importantly intravascular brachytherapy, have emerged as a modality that is available to physicians now and that has gained significant acceptance in the market.
New stents enable additional applications
Drug-eluting coronary stents continue to be the key factor shaping the future of the interventional cardiology market. The focus of all major suppliers of coronary stents has shifted almost completely to the development of drug-eluting devices, led by the Cordis (Miami Lakes, Florida) unit of Johnson & Johnson (New Brunswick, New Jersey) with its Cypher stent. Other suppliers have encountered some challenges in trying to develop technology to compete with Cordis. See Table 2for an overview of current development programs for drug eluting stents. Boston Scientific (Natick, Massachusetts) appears to be closest to Cordis, with Guidant (Indianapolis, Indiana), in a collaboration with Cook (Bloomington, Indiana), also in contention. The initial results of the TAXUS III trial of the Boston Scientific paclitaxel eluting stent fell short of duplicating those of Cordis in its trials with the Cypher stent, although the investigators designed the initial study to test the dosing characteristics of the device, and expect to further improve performance after optimizing the design based on their initial results. Boston Scientific's polymer-coated Express stent was used to treat 30 patients with in-stent restenosis in Taxus III. At six months after treatment, one patient required target vessel revascularization, for a 3.4% rate. A 17.2% rate for major adverse cardiac events (MACE) was reported. In the patient who required target vessel revascularization, retrospective analysis clearly showed that the stent was not positioned over the target lesion, such that the beneficial effect of the drug was lost. In addition, the drug release characteristics of the stent were not optimized, according to the investigators.
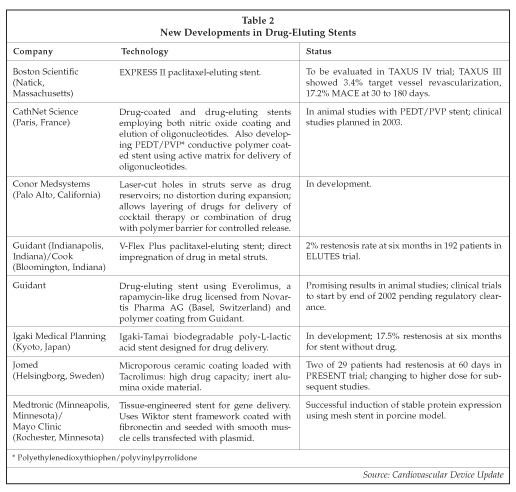 |
The Cypher stent from Cordis, now being evaluated in the RAVEL and SIRIUS trials in Europe and the U.S., continues to set the benchmark of zero restenosis, with some patients who participated in pilot studies now at two years post-implant without recurrence. Some patients with very complex disease have been treated with the Cypher stent, including one with in-stent restenosis who had previously had five stents implanted, as well as diabetic patients and those with small vessels (with diameters of under 2.5 mm). The only adverse events reported so far include death of two patients due to causes believed to be unrelated to the stent, recurrence of a chronic occlusion in a patient whose stent was not completely deployed, and thrombosis in one patient whose clopidogrel treatment was stopped prematurely. The leading investigators involved with the trial of the Cypher stent, such as Patrick Serruys, MD, of the Thoraxcenter (Rotterdam, the Netherlands), were aware that some long-term restenosis had been observed in animal trials with the Sirolimus-eluting stent, but so far no significant tissue re-growth has been detected in the human studies, with an even longer follow-up period than in the animal studies.
In addition to the characteristics of the drug employed, factors influencing the performance of drug-eluting stents include the drug delivery technology used and perhaps the characteristics of the stent itself. A study described by Helmut Schuhlen, MD, at the ACC conference demonstrated that stents with thinner struts produce lower rates of restenosis than those with thick struts. The study compared two versions of the Multi-Link stent from Guidant, as well as the Multi-Link vs. the Cordis BX Velocity, but none of the devices included drug-eluting capabilities. Guidant also is developing the Multi-Link Vision, which includes thin struts plus a cobalt alloy to confer angiographic visibility of the stent.
While zero restenosis may prove to be unachievable in routine clinical practice with patients having complex lesions, experts believe that rates of well under 10% are likely to be the norm once drug-eluting stents reach the market. Other suppliers developing drug-eluting stents include Jomed (Helsingborg, Sweden), Medtronic (Minneapolis, Minnesota) and Abbott Laboratories (Abbott Park, Illinois), the latter in partnership with Biocompatibles International (Farnham, UK), a company that Abbott is now acquiring for approximately $234 million. Some drug/device combinations already have proven inferior, such as Guidant's Actinomycin D eluting stent, a paclitaxel-derivative eluting stent from Quanam Medical (Santa Clara, California) that was under development jointly with Boston Scientific, and a Batimastat-eluting stent from Biocompatibles, demonstrating that selection of the optimal combination of drug, elution platform and stent design is a complex process. The Cordis stent, for example, features a polymer coating from SurModics (Eden Prairie, Minnesota) that is loaded with the drug (Sirolimus), plus a top coating that controls the time-release profile for the drug after implantation. The Jomed stent is one of the more innovative new designs and employs a porous ceramic (aluminum oxide) coating loaded with Tacrolimus. In the initial dose-finding phase of the PRESENT trial with the Jomed stent, two patients developed restenosis at 60 days, and another developed unstable angina. The next phase of the trial will employ a higher dose of Tacrolimus in order to achieve a more complete effect. In other trials, such as the ASPECT trial employing a taxol-eluting stent, higher doses of drug resulted in lower rates of restenosis. However, control of drug delivery is also emerging as an important factor, since some patients have suffered thrombosis in treated vessels after drugs such as Taxol are delivered too rapidly.
Geographic miss is one problem facing cardiologists using drug-eluting stents to treat restenosis. Studies such as the ELUTES trial have encountered restenosis rates of up to 25% if geographic miss occurs, vs. rates of zero for no miss. One innovation intended to address that issue is the Josonics Flex device from Jomed, which will incorporate an intravascular ultrasound (IVUS) imaging capability in the stent delivery catheter to enable more precise targeting of the stent. The Flex will only be available, however, in Europe, since patent issues related to rapid exchange stent delivery systems will not allow it to be sold in the U.S. It will cost about Euro 100 more than a standard pre-mounted stent, vs. a typical cost of Euro 500 to 600 for an IVUS catheter. The Flex also will have applications in direct stenting, a practice becoming increasingly common in Europe, where some suppliers estimate that 50% of all stent procedures are now performed via direct techniques.
New stent designs will allow cardiologists to address a wider group of patients, while achieving improved results. Cordis is developing a new drug-eluting stent system for the treatment of bifurcated lesions, which allows simultaneous stenting of both arms of a bifurcated vessel. Jomed also is developing a new stent, the Easy Access, for treating complex lesions including bifurcations and lesions with side branches. The Easy Access includes a reinforced gap that is positioned over the side branches or the other arm of the bifurcation. Another unique new device, the SLK stent, is under development by Advanced Stent Technologies (Pleasanton, California). The SLK stent is delivered with a two-wire system and includes a large cell in one end of the primary segment of the device (for bridging a side branch) plus a separate tubular segment that is inserted through the cell and into the side branch. Another device providing improved side branch access is the LP2 coronary stent from Boston Scientific. The LP2 was developed by Interventional Technologies (San Diego, California), a company acquired by Boston Scientific in February 2001, and the stent has now been added to Boston Scientific's coronary stent line that also includes the NIR Elite, with additional devices including the Symbiot, a covered stent, and the Express under development.
Another area of development focus in the industry is technologies for the detection of vulnerable plaque and for assessment of the risk of suffering a major adverse cardiac event. If developing plaques are detected early, statin therapy can be used to halt further progression and can even provide a reduction in lipid content of the plaque. Various blood screening tests, such as lipoprotein panels, along with blood pressure testing, have typically been used to identify at-risk patients, but recent studies have shown that improved methods, including new markers such as high-sensitivity CRP and perhaps Tissue Factor assays, along with modified target levels for cholesterol and other lipoproteins, are needed to ensure that all at-risk individuals are identified. High-risk patients can then be tested with non-invasive imaging techniques to determine if vulnerable plaque is present. As discussed by Valentin Fuster, MD, of Mt. Sinai Medical Center (New York) at the ACC gathering, combining ultra-fast CT scans with magnetic resonance imaging is proving useful in elucidating the underlying characteristics of plaque that are associated with the risk of arterial thrombosis resulting in a heart attack. Based on Fuster's studies, the microvascular structure at the base of the plaque, and in particular the density of the vaso vasorum, are better predictors of vulnerable plaque than lipid content or the cap thickness.
Such characteristics can be assessed using imaging systems from suppliers including Philips Medical Systems (Best, the Netherlands), Toshiba America Medical Systems (Tustin, California), Siemens Medical Solutions (Erlangen, Germany) and GE Medical Systems (Waukesha, Wisconsin). High-resolution MRI is proving particularly valuable in allowing changes in plaque characteristics of a specific patient undergoing statin therapy to be assessed over time. A study described by Fuster demonstrated that plaque dimensions could be measured with a reproducibility of 3%, which is sufficient to allow determination of significant progression or regression of atherosclerosis. Electron beam CT, a technology acquired by GE Medical, is used as an initial screen to detect regions with vulnerable plaque, and high-resolution MRI can then be used to analyze plaque characteristics in that region in more detail.
A variety of new blood tests are also under development that promise to improve the ability of physicians to identify individuals at high risk for cardiovascular disease and to guide preventative therapy. Genaissance Pharmaceuticals (New Haven, Connecticut), a leading player in the emerging field of pharmacogenomics, recently announced the discovery of new genetic markers that are linked to changes in levels of LDL and HDL cholesterol and triglycerides in patients undergoing statin therapy. Recent evidence also indicates that levels of CRP, an independent marker of cardiovascular disease risk that indicates vascular inflammation, are modifiable with statin therapy.
Improved techniques for early detection of coronary artery disease are expected to allow preventive therapy to be implemented when it is most effective, avoiding disease progression to a stage when interventional therapy is needed. However, even for those patients who require interventional treatment, the pending introduction of drug-eluting stents promises to provide a minimally invasive, non-surgical treatment option that will in many cases eradicate the problem, at least at the treated site.
Continued erosion of surgeon's turf
As medical and interventional therapies continue to improve, the role of bypass surgery in the treatment of coronary artery disease is expected to decline, at least as a proportion of total procedures. Furthermore, technologies under development by companies such as TransVascular (Menlo Park, California) and others may allow coronary bypass or revascularization to be performed using transcatheter methods rather than surgery. TransVascular has encountered some recent setbacks in the development of the Percutaneous In situ Coronary Venous Arterialization (PICVA) technology, including some deaths in initial safety trials and difficulty in achieving cross-over of blood flow from the arterial system to the coronary venous system. Clinical trials being conducted in Germany with PICVA have been halted temporarily while problems with the technique are resolved. As discussed by Alan Yeung, MD, of Stanford University (Palo Alto, California) at the ACC conference, two deaths occurred as a result of migration of the CrossPoint blocking devices used to re-direct flow in the procedure. Another of the five patients treated suffered a perforation. The company is modifying the blocking devices to use a balloon-expandable design and is improving the guidance capabilities of the system by changing to the Cordis BioSense technology to eliminate the cause of the adverse events. PICVA is presently being evaluated for use in treating patients with no other option for revascularization. Additional studies with PICVA are planned in Germany, at the Cleveland Clinic Foundation (Cleveland, Ohio) and at Beth Israel Medical Center (Boston, Massachusetts).
Another strategy for revascularization being pursued by TransVascular is the use of transvenous technology for delivery of angiogenesis agents. The company is evaluating the delivery of fibroblast growth factor beta (beta FGF) to promote angiogenesis in the coronary tissues. The transvenous technology improves delivery of drugs threefold, to a level of 12% of the injected dose vs. only 4% with direct injection. The company plans to initiate a trial to study the treatment of patients with congestive heart failure later this year.
Patients with chronic total occlusions represent a significant proportion of cases now referred to surgery rather than interventional treatment. According to Matthew Selmon, MD, of the Cleveland Clinic Foundation, a co-founder of LuMend (Redwood City, California), about one-third of all angiograms show evidence of chronic total occlusions (CTOs), and the presence of a CTO is the most common contraindication for interventional treatment. The use of stents is allowing improved outcomes to be achieved when treating CTOs, and drug-eluting stents are expected to allow even better outcomes to be achieved. However, recanalization of CTOs via interventional techniques is challenging, with a significant rate of procedural events such as perforation when using various types of stiff guidewires to open the occlusion, and high rates of restenosis are often observed after treatment. As shown in Table 3, a number of new devices are under development for treating CTOs, and some have recently become available in the U.S. and/or Europe. Survival of CTO patients who are treated is significantly improved (to about 80% vs. 65% if the occlusion is not opened). The new devices for CTO treatment are designed to allow a higher percentage of CTOs to be treated vs. the approximately 40% to 70% of combined functional and complete CTOs that are recanalized now. The LuMend FrontRunner, the only FDA-cleared device so far, takes advantage of the different mechanical properties of plaque vs. the vessel wall to help guide the device through the plaque. Disadvantages of the device include the requirement for a large guide catheter, some continued incidence of perforations, cost and lack of successful recanalization in about 44% of the attempted cases.
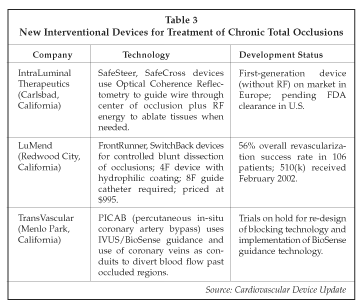 |
The IntraLuminal (Carlsbad, California) device uses optical reflectometry to differentiate between plaque and the vessel wall, with red and green lights showing the operator the presence of vessel tissue and plaque respectively. Because some occlusions continue to resist mechanical penetration by the wire, a second-generation version has been developed that incorporates a radiofrequency source to ablate firm regions of plaque. TransVascular also is evaluating its percutaneous coronary venous bypass technology (PICAB) for CTO treatment, which allows connection to a coronary vein that serves as an alternative conduit to bypass the occlusion. The PICAB technology is at an early stage of development for this application, however.
Treatment of diseased saphenous vein grafts is another growing application for interventional cardiology. A large number of patients who have had a prior bypass procedure suffer failure of the bypass graft. Between 12% and 20% of the saphenous vein grafts implanted for coronary bypass typically occlude within the first year, and about 2% to 4% occlude each year thereafter. Interventional treatment of failed grafts using conventional balloon and stent technology has proven challenging due to the often ulcerated state of the vessel and typical diffuse nature of the occlusions. A PTFE-covered stent introduced by Jomed has proven useful for treating dissections in saphenous vein grafts, although, as discussed by Jonathan Tobis, MD, of UCLA Medical Center (Los Angeles, California), the device is not a complete solution. In principle, the stent covering prevents debris from the vessel from breaking loose and reaching the blood stream. However, a large amount of debris is typically generated when treating saphenous vein grafts, with macroscopic debris observed in half of all patients, based on a study conducted with the PercuSurge embolic capture device. Even with the Jomed covered stent, some embolization and thrombosis continue to occur according to Tobis, although there is certainly a benefit. A number of other devices also are used to help clear diseased grafts prior to stent placement, including the AngioJet XMI catheter from Possis Medical (Minneapolis, Minnesota), and the X-Sizer, a thrombectomy device developed by EndiCOR Medical (San Clemente, California) and now marketed by Microvena (White Bear Lake, Minnesota). Yet another device, the Aegis Vortex System, uses aspiration atherectomy to both create a new lumen through an occluded graft as well as to aspirate thrombus and plaque, thus minimizing the risk of distal embolization. In a feasibility study involving 12 patients, particles were aspirated in all cases, and no adverse events occurred.
In-stent restenosis options expand
Devices for treatment of in-stent restenosis comprise yet another rapidly growing new segment of the interventional cardiology products market. A variety of approaches are under study, and some, such as intravascular brachytherapy, are available in the global market (Table 4). The Novoste (Norcross, Georgia) Beta-Cath is the leading brachytherapy system in use worldwide for the treatment of in-stent restenosis, with reported revenues of almost $70 million in 2001. According to experts discussing in-stent restenosis treatments at the ACC meeting, intravascular brachytherapy is now being used successfully in a large number of institutions in the U.S. and Europe. Geographic miss remains an important issue, particularly with beta irradiation systems. However, sales for Novoste, the leading supplier of beta irradiation devices for intravascular use, increased over 10-fold from 2000 to 2001. Restenosis rates for the treatment of in-stent restenosis using the Novoste system were reported at about 29% versus 45% in vessels not receiving radiation treatment.
 |
However, the most promising results for in-stent restenosis treatment have been obtained using drug-eluting stents, specifically the Cordis Cypher stent. As discussed by Martin Leon, MD, of the Cardiovascular Research Foundation (New York), initial studies of in-stent restenosis treatment using the Cypher stent have shown that restenosis can be completely eliminated provided the stent is placed properly to avoid geographic miss. The treated patients included some who had failed vascular brachytherapy, as well as some with chronic total occlusions. Aggressive use of anti-coagulant therapy (Plavix) is important, as one patient who stopped Plavix treatment after receiving a Cypher stent suffered thrombosis. Nevertheless, the results indicate that drug-eluting stents may be the ultimate solution to in-stent restenosis as they appear to be for prevention of restenosis in de novo lesions. The TAXUS III trial, using a paclitaxel-eluting stent from Boston Scientific, has also achieved promising results in treating in-stent restenosis patients.
There may also be a role for certain new types of atherectomy devices in the treatment of in-stent restenosis, particularly for patients who have developed significant build-ups of neointimal tissue within the stent. Promising results have been obtained with the Helixcision helical atherectomy system from Prolifix Medical (Sunnyvale, California). Other atherectomy devices also can be employed, such as Guidant's Directional Coronary Atherectomy system, the Rotablator from Boston Scientific and the X-Sizer catheter. However, there are concerns regarding damage to the stent when using techniques such as DCA or the Rotablator, according to Andrew Carter, MD, of Stanford University (Palo Alto, California). The Prolifix device exhibits excellent tracking as well as efficient debulking, according to Carter.
