Research carried out by a team at St. Jude Children's Research Hospital in Memphis highlights the importance of carefully assessing the mouse model you plan to use before starting preclinical medical research.
The team discovered that the specific genetics of two mouse models commonly used in research on sickle cell disease (SCD), the Berkeley and Townes models, meant that when they tried to disrupt part of the gene encoding hemoglobin using CRISPR-Cas9 gene editing to trigger the production of fetal hemoglobin, this was largely unsuccessful.
"We started trying to create these mutations and, as we did, we realized that something wasn't quite right with this model [Berkeley], the cells were not responding like normal human cells when we added them," Kaitly Woodard, now based at Novartis, told BioWorld Science. Woodard's PhD thesis was based on this work and she is first author of the paper describing the study published in the journal Disease Models & Mechanisms.
In humans, the type of hemoglobin produced by the body changes during development with the early embryo producing one kind, the mid-late embryo another and then after birth a switch occurs from fetal to adult hemoglobin.
Fetal hemoglobin can bind to oxygen more strongly, allowing the fetus to extract the maximum amount of oxygen from the mother's blood during development. In a newborn baby, the type of hemoglobin gradually switches from fetal to adult over the first few months of life with levels of fetal hemoglobin remaining high until 4 months of age.
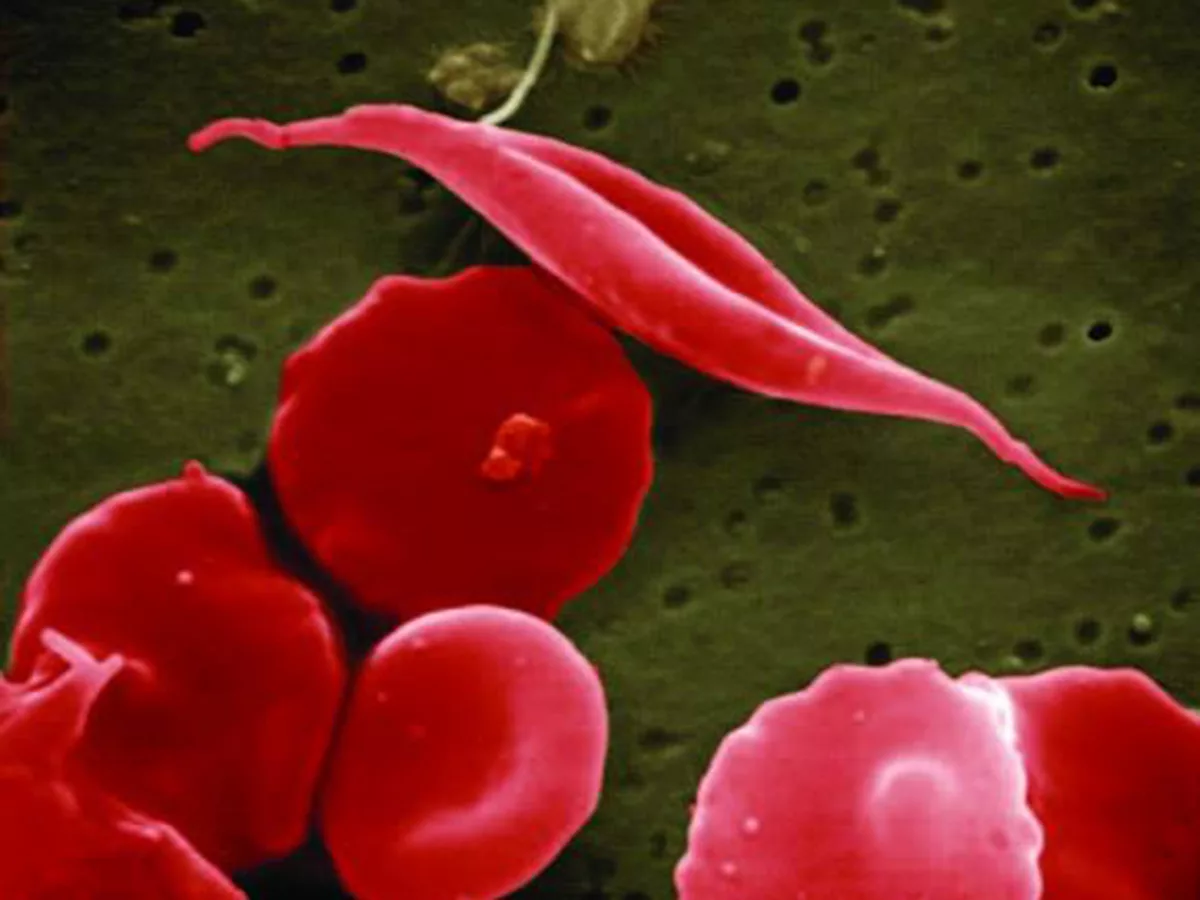
People with SCD inherit a recessive mutation from both parents in the HBB gene, which is responsible for producing hemoglobin to carry oxygen in red blood cells. These mutations mean that the red blood cells become rigid and form a 'sickle' shape under certain circumstances causing blood vessel blockages, pain and other symptoms. These individuals tend to show symptoms only at around 5-6 months of age when fetal hemoglobin is low or nonexistent.
Triggering the production of fetal hemoglobin, for example, using hydroxyurea, has shown benefits in individuals with SCD. Woodard and group leader Mitchell Weiss, a professor and clinician at St. Jude's, were testing whether they could induce the production of red blood cell fetal hemoglobin in the SCD mouse models as a possible therapeutic strategy for people with SCD.
"If we made the modification in the Berkeley stem cells, and we put them back into the mice, the mice died... if we did it in the Townes mouse, the mice survived and the edited cells induced fetal hemoglobin, but it was a really weak induction of fetal hemoglobin," Weiss told BioWorld Science.
When their strategy failed, the researchers decided to look more closely at the genetics of the transgenic mouse models to see if this could explain the problem. When they found little information on the exact genetics of the models in the literature, they decided to investigate more closely themselves.
"Something that's been kind of assumed for years is that creating transgenic mice by doing random injection into embryos will give you multiple copies of whatever gene you're injecting. We were able to use the newest sequencing technology to actually prove this, and show how many copies there were within that Berkeley mouse," explained Woodard, adding that over 20 copies of the sequence they were trying to edit were found in these mice.
"When we were trying to edit the cells with CRISPR-Cas9, which creates double-stranded breaks, we ended up creating more than 20, and sometimes 40, double-stranded breaks. It just was too toxic for the cells so that that strategy was never going to work in that mouse. But because we didn't know the extent of the model, we weren't aware of it," she added.
"If you create one double-stranded DNA break, the cell can repair it," noted Weiss. "But if you create too many double-stranded DNA breaks, what happens is the cells get very unhappy. They activate DNA damage responses, some of which are p53-mediated, and they can have massive chromosomal rearrangements."
In contrast, the Townes mice did not have multiple copies of the mutated human hemoglobin gene, but they were missing some regulatory elements of sequence needed for the gene to function properly and produce high levels of fetal hemoglobin.
Weiss says this is because the model was created before in-depth knowledge of the gene and its regulators. "They thought they were putting in everything that might be needed for regulation, because it was a long time ago," he explained.
"In the last 5 years, we and other people have been busy mapping regulatory elements to control this developmental switch, and some of those regulatory elements fall outside of the promoter. We were able to show that some of those regulatory elements are not present in the mice."
Take-home messages
The Berkeley mouse model of SCD was created in 1997 and the Townes model in 2006. Both have been widely used in sickle cell research. When they searched the literature, Weiss and Woodard found little information on the specific genetics of the two models. This is likely because of the age of the models, particularly in the case of the Berkeley SCD mouse.
"Most people understand [now] that if you are going to publish a genetic model, you need to show what you've created, because you can make a lot of mistakes. But these mice were made a long time ago. And the requirements for reporting weren't as stringent. Nor were the methods to figure this out," explained Weiss.
The researchers hope their study will help other scientists working with the same models to avoid making the same assumptions and they encourage others to carefully check the genetics of the transgenic model they plan to use before proceeding with preclinical studies. "What you need to ask is, am I using the right model for the question that I'm asking?" said Weiss.
Weiss added that with the frequent use of human cell lines in preclinical research into conditions such as SCD it is unlikely that a negative result in mice would stop a potentially viable therapy reaching human trials, but says it's good to be aware of the limitations of the model you are using to avoid this. "I can't think of an example where a result arising from these mice discouraged somebody from making a drug. But it might, it's a possibility," he commented.