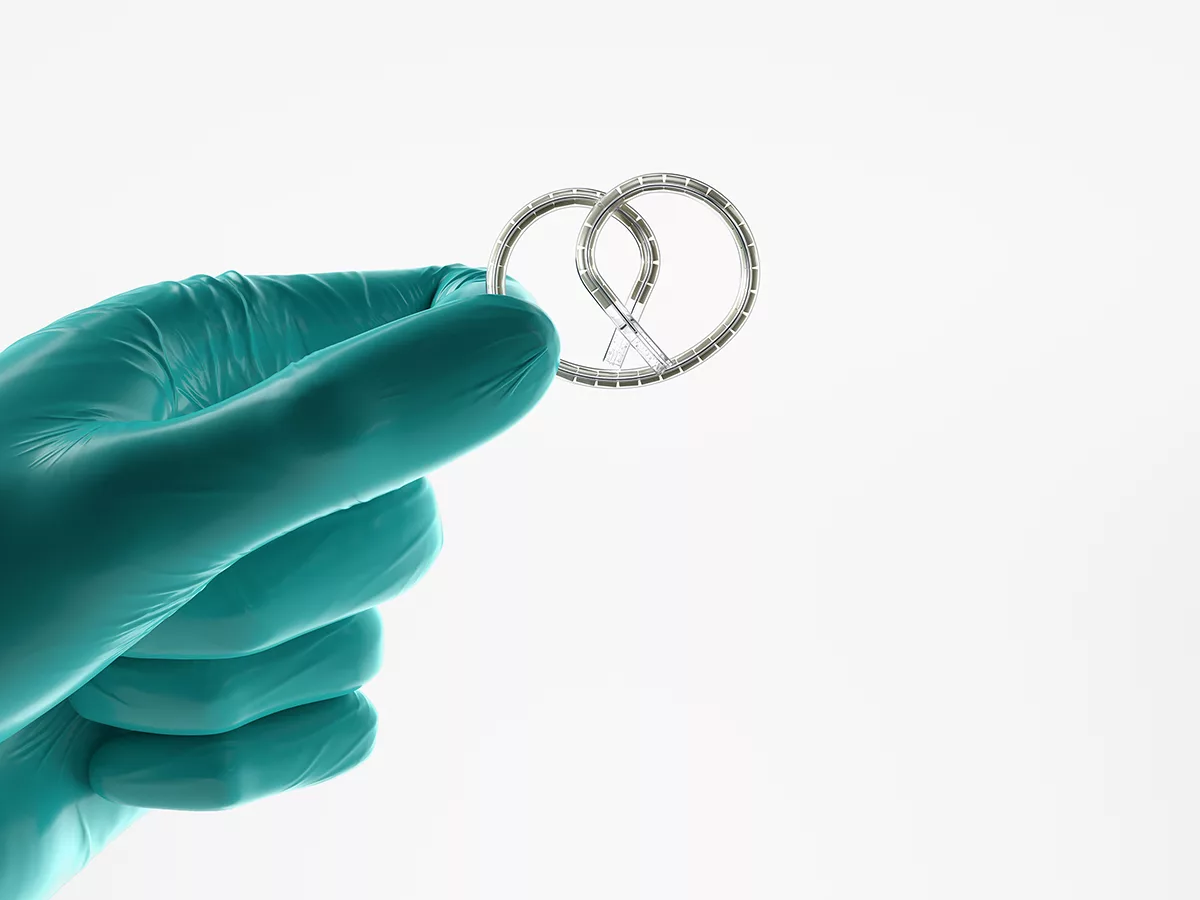
Less than two months after receiving priority review status for an NDA, Johnson & Johnson won U.S. FDA approval of Inlexzo, its intravesical gemcitabine releasing system previously known as TAR-200, to treat adults with Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.
It offers bladder cancer patients a new option prior to a radical cystectomy, or bladder removal surgery, by delivering cancer medication into the bladder, where it is released for three weeks per treatment cycle. Patients are administered the drug for up to 14 cycles with a health care professional placing it within a few minutes, without anesthesia or further monitoring, into the bladder with a co-packaged urinary catheter and stylet.
Raritan, N.J.-based J&J acquired the therapy in 2019 through its buyout of Taris Biomedical LLC, of Lexington, Mass., and through technology licensed from the Massachusetts Institute of Technology. The treatment system gained breakthrough therapy designation in December 2023 and priority review in July 2025, meaning a decision would typically come within six months of the FDA accepting the application. J&J initiated the NDA submission in January, noting that the FDA was reviewing Inlexzo through the real-time oncology review program.
“In an area that has seen little progress for more than 40 years, Inlexzo delivers a first-of-its-kind breakthrough innovation with a bright future ahead,” said Jennifer Taubert, executive vice president and worldwide chair of J&J’s Innovative Medicine unit.
The approval is based on data from the Sunrise-1 phase IIb trial, which showed 82% of patients with BCG-unresponsive NMIBC achieved a complete response (CR; 95% CI), with 51% of them maintaining the CR for at least a year. The most common adverse reactions, occurring in at least 15% of patients, included urinary frequency, urinary tract infection, dysuria, micturition urgency, decreased hemoglobin, increased lipase and others.
Sunrise-1 was a single-arm, open-label trial that evaluated Inlexzo as a monotherapy.
NMIBC with CIS accounts for about 10% of patients with NMIBC, with BCG – a weakened form of the bacteria found in tuberculosis treatment – being the current standard of care. Radical cystectomy is the next step for those who fail, leading to high morbidity and an adverse life impact, with a 3% to 8% mortality rate post-surgery.
Inlexzo is expected to compete with CG Oncology Inc.’s cretostimogene grenadenorepvec in high-risk NMIBC unresponsive to BCG with CIS. CG plans to submit a BLA later this year. Pfizer Inc. and Urogen Pharma Ltd. also have candidates in development.
Animation of Inlexzo (gemcitabine intravesical system). Credit: Johnson & Johnson