Evoke Pharma Inc. enrolled the first patient in the phase III trial of EVK-001, its intranasal formulation of metoclopramide, to treat symptoms associated with acute and recurrent diabetic gastroparesis in women. Founded in 2007, the Solana Beach, Calif.-based specialty pharma has remained laser-focused on the development of its only product, which the FDA agreed to review on the basis of a single pivotal trial.
Evoke is conducting a parallel phase III trial of EVK-001 in men with diabetic gastroparesis, which will provide additional data but will not form the basis of the company's new drug application submission, according to David Gonyer, Evoke's co-founder, president and CEO.
The four-week, multicenter, placebo-controlled, double-blind, parallel-group Phase III study is expected to enroll 200 patients at approximately 60 U.S. sites. Patients will be randomized to metoclopramide nasal spray 10 mg or placebo, 30 minutes before meals and at bedtime. The primary outcome measure is change from baseline at four weeks in patient-recorded outcomes. The study also is measuring population pharmacokinetics, according to Thomson Reuters Cortellis Clinical Trials Intelligence (CTI).
"We're looking to confirm our phase II data, which had very good results," Gonyer told BioWorld Today. In January, phase IIb data published in Neurogastroenterology & Motility showed that intranasal, systemic delivery of metoclopramide was more effective in managing symptoms of diabetic gastroparesis than the marketed oral formulation of metoclopramide (Reglan, Wyeth LLC).
Top-line data are expected by mid- to late 2015, according to Gonyer, who said the company is prepared to submit a filing quickly – potentially, by year-end 2015.
Gastroparesis is a disorder in which the stomach is delayed in emptying its contents to the small intestine, despite the absence of an obstruction. Symptoms include nausea, vomiting, abdominal pain and bloating.
The condition is difficult to treat because unpredictable gastric emptying and vomiting can interfere with absorption of food and medications. For individuals with diabetes, symptom flares can affect blood glucose control and lead to complications that may require hospitalization. Some 12 million to 16 million adults in the U.S. are affected with the condition, with women accounting for more than 80 percent of the patient population, according to Evoke.
"The idea we have is to deliver intranasally and bypass the stomach so the drug goes directly to the bloodstream and you can have the desired effect," Gonyer said. "In a disease where pills and food are just sitting in the stomach, pills just don't make any sense."
The company holds exclusive rights to intellectual property associated with EVK-001 that provide U.S. patent protection through 2021.
'WE'LL DO WHAT'S RIGHT FOR THE SHAREHOLDERS'
Despite the severity of symptoms associated with gastroparesis, the indication has attracted little attention from biopharmas. Oral metoclopramide is the only drug approved in the U.S. to treat the condition.
That drug has caused headaches for Pfizer Inc., which acquired Wyeth, when the Alabama Supreme Court ruled last year that Pfizer was on the hook for injuries caused even by generic versions of Reglan if it failed to warn about risks associated with the drug's long-term use. In Wyeth Inc. v. Danny Weeks, Weeks, who developed tardive dyskinesia after years of using generic metoclopramide, alleged Wyeth and Pfizer "falsely and deceptively misrepresented or knowingly suppressed facts" about the drug. Pfizer faces about 3,500 similar cases nationwide in a battle that is could turn on the FDA's proposed rule to enable generic drugmakers to change their labels independently of the brand drug. (See BioWorld Today, Jan. 15, 2013, and March 21, 2014.)
Cortellis CT identified 28 trials in which gastroparesis or diabetic gastroparesis is the primary indication. Motilin receptor agonists represent the top mechanism of action for drugs under evaluation in the indication. Among those, Glaxosmithkline plc's oral compound GSK-962040 (camicinal) is being studied in type 1 diabetics with gastroparesis and in patients with Parkinson's disease who exhibit delayed gastric emptying. Chugai Pharmaceutical Co. Ltd. also has a compound, mitemcinal, in phase II development in gastroparesis. (See chart below.)
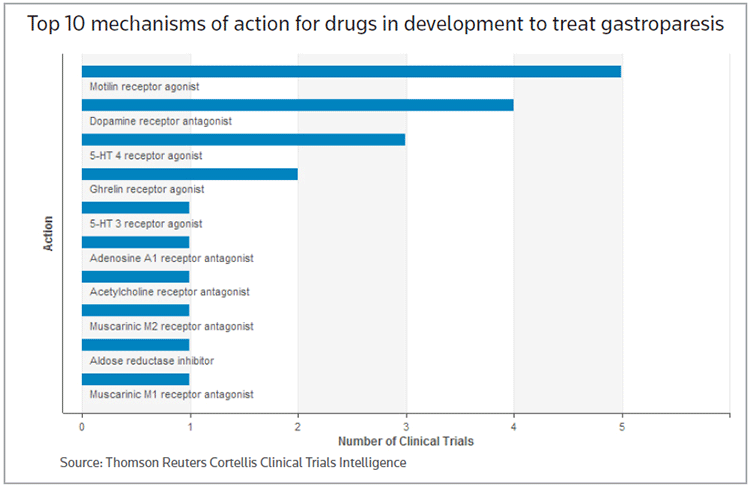
EVK-001 represents three of the four trials for metoclopramide, a dopamine receptor antagonist. Questcor Pharmaceuticals Inc., which recently agreed to a takeover by Dublin-based Mallinckrodt plc, also studied a nasal spray version of metoclopramide, known as Emitasol, but the compound never moved beyond the South Korean market.
Among the 5-HT 4 receptor agonists studied in the indication is Theravance Inc.'s velusetrag, which is being evaluated in a collaboration with Italian pharma Alfa Wassermann SpA in gastric emptying in patients with diabetic or idiopathic gastroparesis. Rhythm Pharmaceuticals Inc. also is evaluating RM-131 in diabetic gastroparesis.
Most of the remaining two dozen trials are being conducted at academic medical centers, with Evoke the only company that has moved a gastroparesis candidate into phase III.
The indication has thwarted other biopharmas, with a dozen trials terminated in recent years. Among the notable losers was Tranzyme Pharma Inc., which was pummeled when top-line data from two phase IIb trials of its candidate, TZP-102, missed their primary efficacy endpoint. (See BioWorld Today, Nov. 16, 2012.)
In 2013, Evoke closed a modest initial public offering, raising just $25.2 million, but "we have enough money to finish the phase III and get to the data," Gonyer said. (See BioWorld Today, May 29, 2013.)
The company has an extraordinarily low cash burn, reporting $24 million in cash and equivalents as of Dec. 31. That gives Evoke the flexibility to consider multiple commercialization strategies in the U.S. – its only target market – ranging from a deal with a big pharma partner to taking the product forward on its own.
"We'll do what's right for the shareholders," Gonyer said.
On Tuesday, those shareholders were bullish about the phase III start, lifting the company's shares (NASDAQ:EVOK) $1.43, or 18.2 percent, to close at $9.29.
