Effective treatments for sickle cell disease (SCD) have been 100 years in the making but are now nearing the finishing line. Several companies are advancing innovative potential treatments for the disease and its associated complications. That will come as welcoming news given the fact that only one FDA-approved drug is available and none have yet hit the market that treat the associated ongoing vaso-occlusive crisis (VOC) or underlying ischemia and infarction that reduce the average life expectancy of people suffering from the disease.
SCD is an inherited blood disorder of misshapen red blood cells with an abnormal oxygen-carrying pigment called hemoglobin S, resulting in chronic anemia. Painful VOCs are responsible for more than 70,000 hospitalizations of SCD patients per year in the U.S., with an average stay of about six days.
One company well advanced with its SCD therapy is San Diego-based Mast Therapeutics Inc. In April, the company reported patient enrollment in its pivotal EPIC study of lead product candidate vepoloxamer (MST-188), a purified form of a nonionic, triblock copolymer (poloxamer 188), had moved past the halfway point and, according to the CEO Brian Culley, the firm is “on track to announce top-line data in the first quarter of next year,” an event that will be closely watched by patients and investors alike.
Vepoloxamer is designed to lower the viscosity of SCD patients’ blood during a crisis by lowering adhesion and improving the malleability of cells, improving blood flow and thereby shortening the duration of the crisis.
CRISIS REDUCTION
The EPIC study is designed to demonstrate that vepoloxamer reduces the duration of VOC in patients with SCD. The duration of VOC is being measured from the time a subject is randomized to the time at which the subject receives the last dose of parenteral opioid analgesic for the treatment of VOC prior to hospital discharge. The plan is to enroll a total of 388 patients, ages 4 to 65. The study also will compare the rates of re-hospitalization for VOC and occurrence of acute chest syndrome between the treatment and control groups.
In May, Mast initiated an open-label extension study, EPIC-E, to evaluate repeat dosing of vepoloxamer in patients who have completed the EPIC trial and are hospitalized for a subsequent VOC. (See BioWorld Today, May 27, 2015.)
EPIC-E is being conducted to support Mast’s planned FDA new drug application for vepoloxamer in SCD.
Also in late-stage testing with its candidate product is Glycomimetics Inc., of Gaithersburg, Md., via partner Pfizer Inc. The pharma company reported late June that it had dosed the first patient in the RESET (Rivipansel: Evaluating Safety, Efficacy and Time to Discharge) study – a phase III trial assessing the efficacy and safety of rivipansel for the treatment of VOC in patients hospitalized with SCD, ages 6 or older. That event triggered a $20 million milestone payment from Pfizer. Glycomimetics received another $15 million milestone payment from Pfizer in May last year.
The study is planning to enroll at least 350 individuals who are hospitalized for VOC and the primary endpoint will be time to readiness-for-discharge. Key secondary endpoints will include time to discharge, cumulative intravenous (I.V.) opioid consumption and time to discontinuation of I.V. opioids.
Glycomimetics successfully completed an upsized IPO last year, pricing 7 million shares of its common stock at a price of $8 per share. Hoping to repeat that performance this year is South San Francisco-based Global Blood Therapeutics Inc., which filed its registration documents for an IPO.
Its initial product candidate, GBT440, is being developed as a once-daily, oral therapy for SCD. It is currently being tested in a phase I/II trial. The company said its product targets the underlying mechanism of red blood cell sickling, which it believes provides the potential to treat SCD rather than only its associated symptoms.
Emmaus Life Sciences Inc., of Torrance, Calif., has an oral pharmaceutical-grade L-glutamine (PGLG) for the treatment for sickle cell anemia. Results of a phase III trial were presented in December 2014 at the American Society of Hematology meeting.
The trial studied the efficacy and safety of PGLG for SCD in 230 pediatric patients as young as 5 and adults. Study participants were randomized to receive daily PGLG (152 patients) or placebo (78 patients) for 48 weeks, after which treatment levels were tapered to zero.
Researchers observed that patients who received PGLG experienced fewer painful crises (three vs. four events during the study period, a 25 percent reduction) and a longer time to a pain crisis than patients receiving placebo. Treated patients were also less likely to be hospitalized for their condition (two vs. three events during the study period, a 33 percent reduction) and spent less time in the hospital for those events (6.5 vs. 11 days, a 41 percent reduction) than those receiving placebo. The percentage of patients experiencing acute chest syndrome, a severe complication of SCD, was less than half among the PGLG group as compared to the placebo group (11.9 percent vs. 26.9 percent, or 58 percent fewer cases).
FULL PIPELINE
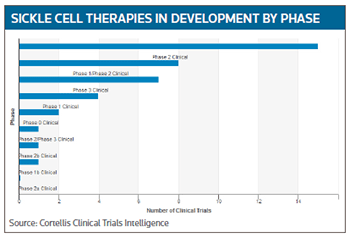 Among the ongoing trials in sickle cell disease (See “Sickle Cell Disease Trials by Phase,” below) is Aes-103 is a first-in-class, oral, small-molecule compound (5-hydroxymethylfurfural), being developed by Aesrx LLC, of Newton, Mass. Early research showed it may work by binding to hemoglobin and increasing oxygen affinity and stabilization, reducing the sickling of red blood cells which, in turn, may reduce sickling-related outcomes such as VOC, pain, severe anemia and fatigue.
Among the ongoing trials in sickle cell disease (See “Sickle Cell Disease Trials by Phase,” below) is Aes-103 is a first-in-class, oral, small-molecule compound (5-hydroxymethylfurfural), being developed by Aesrx LLC, of Newton, Mass. Early research showed it may work by binding to hemoglobin and increasing oxygen affinity and stabilization, reducing the sickling of red blood cells which, in turn, may reduce sickling-related outcomes such as VOC, pain, severe anemia and fatigue.
The Aes-103 program is in a phase II trial as part of an ongoing collaboration with the NIH’s National Center for Advancing Translational Sciences through its Therapeutics for Rare and Neglected Diseases program. (See BioWorld Today, Sept. 9, 2013.)
Baxter International Inc., of Deerfield, Ill., has gained rights to Aes-103, through its acquisition of Aesrx last year.
Commenting on the transaction, Ludwig Hantson, president of Baxter Bioscience, said the program is “complementary to our established experience in hemophilia and supports our goals to raise the bar for care of patients with a range of blood-related disorders.”
Oklahoma City-based Selexys Pharmaceuticals Corp. is testing the anti-P-selectin monoclonal antibody SelG1, with or without hydroxyurea – the only FDA-approved SCD treatment. SelG1 prevents certain blood cells from binding to one another and to the blood vessel walls.
In August 2013, the company reported it had started enrolling patients in SUSTAIN, a phase II study to assess safety and efficacy of SelG1 in SCD patients with sickle cell-related pain crises. The trial will randomize approximately 174 patients to receive high-dose SelG1, low-dose SelG1 or placebo in the presence or absence of hydroxyurea therapy. The study will be conducted in approximately 60 centers throughout the U.S.
GENE THERAPY
Since SCD is a genetic disease causing amino acid substitution on the beta-chain of hemoglobin, Bluebird Bio Inc. is using a gene therapy approach toward the disease. In June, the company reported early safety and efficacy data in the first patient with severe SCD treated with Lentiglobin BB305 in the HGB-205 study, which also is treating beta-thalassemia patients, at the European Hematology Association.
The data showed that the proportion of anti-sickling hemoglobin being produced by the first-ever patient with severe SCD treated with gene therapy is rising steadily and accounted for 45 percent of all hemoglobin production at the patient’s six-month visit post-drug infusion; that is above the 30 percent threshold expected to potentially achieve a disease-modifying clinical effect.
As of May, the patient had been free of transfusions for more than three months without complications or hospitalizations for SCD-related events post-transplant, and with improvement in hemolysis markers.
