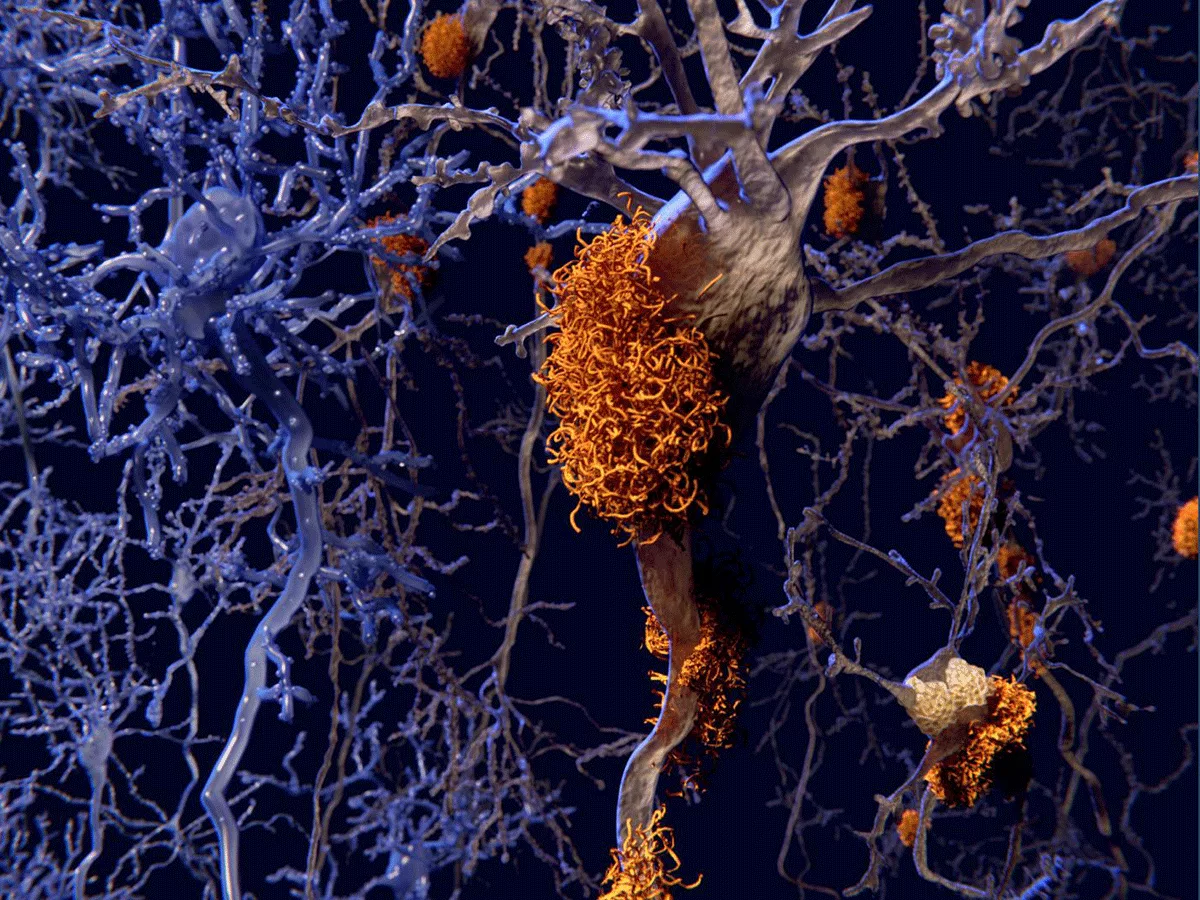
Alzheimer's disease (AD) currently afflicts over 6 million Americans with expected financial burdens for long-term care and hospices for this population exceeding 350 billion.
These numbers threaten to bankrupt Medicare and Medicaid and to overwhelm the nation's long-term care facilities, to say nothing of the toll it will take on caring families.
In spite of having identified ApoE isoforms (particularly ApoE4 homozygous) as the strongest genetic link to late-onset AD nearly 30 years ago, at this point there are still no therapeutics that can reverse AD's cognitive decline.
However, in 2017 researchers discovered that the elimination of brain ApoE can prevent Alzheimer's-like tau pathology and associated neurodegeneration in mice models, suggesting that the clinical reduction of ApoE may be a desirable therapeutic target goal.
Now, the same researchers have taken these observations one critical step further to report in the June 21, 2021, online issue of Neuron that overexpression of the LDL receptor (LDLR) can reduce ApoE to prevent tauopathy-associated neurodegeneration in mouse models.
The study supports the idea that targeting of the LDLR in the brain is worth considering as a therapeutic approach. The study's principal investigator, professor Holtzman, pointed out that while LDLR has been a major target for cardiovascular disease for a very long time, this study is one of the first examples focusing on brain-specific LDLR, rather than peripheral LDLR.
David Holtzman, professor and chair of the department of neurology, scientific director of the Hope Center for Neurological Disorders, and associate director of the Knight Alzheimer's Disease Research Center at Washington University School of Medicine, clarified one of the common misunderstandings of ApoE genetics today.
Generally, most people think of ApoE2 as protective against Alzheimer's, ApoE3 as neutral and ApoE4 isoform homozygosity as bad, but in fact his laboratory has determined that these interpretations are based on their pathogenic contributions relative to each other. However, his experimental observations suggest that the presence of any brain ApoE can contribute to greater tauopathy. Genetic elimination of brain ApoE prevented tau pathogenesis in a previously published study.
The work of Holtzman and his team has focused on understanding why ApoE4 is the strongest genetic risk factor for AD. He spent most of the first part of his career focused on amyloid deposition controlled by ApoE isoforms, but only very recently have they begun to appreciate exactly how ApoE contributes to neurodegeneration caused by tauopathy.
In their previous study, Holtzman told BioWorld Science, "tauopathy mice develop tau pathology with a lot of damage in the brain with loss of neurons, loss of synapses, and the brain degenerates, but when ApoE was removed, there was no damage. This was amazing! After we observed this, we tried to think of ways to lower ApoE in the brain to see if that would cause a similar protective effect. That is why we started working on overexpressing LDL-receptor, because while there are several receptors for ApoE, in the body the one that really controls the absolute levels of ApoE protein the most, is the LDL-receptor."
Holtzman explained that blood lipoproteins have different functions depending on the nature of the particle. For example, the major lipoprotein in blood LDL is ApoB, which is also abundant in very-low-density lipoprotein (VLDL). Lipoproteins function to deliver cholesterol, cholesterol esters and/or phospholipids to different cells, while high-density lipoprotein (HDL) uses ApoA1 protein primarily to function in reverse transport, moving these same categorical molecules to the liver for redistribution/excretion.
However, Holtzman pointed out that the brain is different because it only has HDL. Moreover, the main component protein of brain HDL is ApoE. The role of these HDLs in the interstitial fluid of the brain is unclear. Brain HDL may be functioning in part to deliver cholesterol, cholesterol esters and/or phospholipids. But Holtzman suspects that one of the main roles of ApoE may actually also be in the removal of excess cholesterol, as opposed to delivering it.
Holtzman pointed out that blood lipoprotein proteins cannot get into the brain. Rather, neurons can synthesize their own cholesterol de novo. But if there is an injury, such as the demyelination that occurs in multiple sclerosis or other diseases, brain cholesterol needs to be reutilized. ApoE may be involved in such redistribution processes.
The data suggest that a small-molecule approach to increasing LDLR expression may ultimately be therapeutic in reducing diseases involving tauopathies, such as AD, frontotemporal dementia, progressive supranuclear palsy or chronic traumatic encephalopathy. Going forward, the investigators plan to identify small molecules that can increase LDLR.