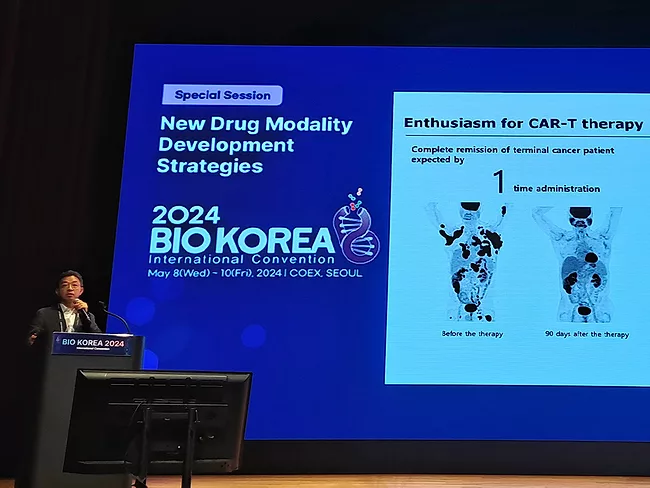
Analysis and data insight, BioWorld Asia
China’s CAR T market comes of age
CAR T pipelines bloom to treat world’s largest cancer population
Read MoreChina’s CAR T market comes of age
China’s investigator trials accelerate competitive CAR T development
Read MoreBiopharma regulatory actions and approvals March 2024
US FDA makes history with 30 drugs approved in March
Read MoreBiopharma regulatory actions and approvals February 2024
11 US approvals in February include biosimilar, treatments for frostbite, HIV
Read MoreBiopharma deals February 2024