It’s amazing what a difference a decade can make.
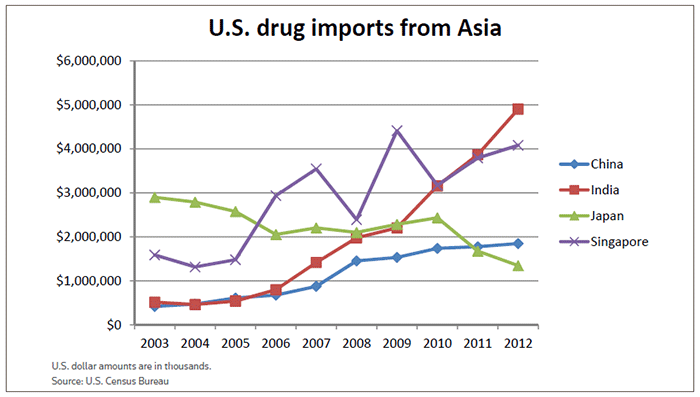
In 2003, Japan and Singapore were the only countries in the Asia-Pacific region that exported more than $1 billion worth of drugs to the U.S. Japan shipped about $2.9 billion worth of “medicinal, dental and pharmaceutical preparations,” and Singapore exported drug products valued at nearly $1.6 billion, according to the U.S. Census Bureau.
A decade later, India had surpassed both countries, even though Singapore’s drug exports to the U.S. had jumped 157 percent to nearly $4.1 billion. And China shot past Japan, which saw its drug exports drop 53 percent to a 10-year low of about $1.35 billion.
The turning point occurred in 2006-2007. That’s when Japan’s exports to the U.S. began to slip and drug exports from China, India and Singapore started their upward climb.
At the heart of this shift was a change in Japan’s regulatory policy that allowed Japanese biopharma companies to outsource the manufacture of their drugs, said Akiko Hihara, a managing editor for Cortellis Regulatory Information. (Editor’s Note: Cortellis and BioWorld Asia are both part of Thomson Reuters.)
Once the revised Pharmaceutical Affairs Law was implemented in 2005, Japanese drugmakers sent entire manufacturing processes to contract manufacturers and plants they had established elsewhere in Asia. As a result, the region’s manufacturing base shifted from Japan to China, India and other emerging Asian countries, Hihara told BioWorld Asia.
At the same time, China and India began investing more heavily in biopharma R&D, and both countries revised their own policies to support technological upgrades and specialization, said Jianhui Zheng, a managing editor for Cortellis.
This new focus and the countries’ adoption of the World Trade Organization’s Trade-related Aspects of Intellectual Property Rights led to regulations favoring innovation and exports in China and India, as well as a push for drug manufacturers to improve their quality control systems.
The changes made drugmakers in China and India choice targets for strategic alliances and direct investments as Japanese companies and global players saw them as a viable manufacturing alternative and a doorway to expansive markets. As time passed, those investments have enhanced the capabilities of drugmakers in China and India – not only in manufacturing, but also in technology and innovation, Zheng told BioWorld Asia.
At the same time, Japan’s drug manufacturing has suffered, as the country now imports more drugs than it exports, Hihara said.
While the policy changes have hurt Japan’s manufacturing base, they have helped China and India make great strides, as the Census Bureau figures show. India, for instance, is now Asia’s largest supplier of U.S. drugs, exporting about $4.9 billion in drugs in 2012. That’s an 851 percent increase over the $515.5 million it sent to the U.S. in 2003.
China’s drug exports to the U.S. increased 341 percent over that 10-year period – from $418.9 million in 2003 to more than $1.8 billion in 2012.
While a growing number of the drugs imported into the U.S. are from Asian countries, Ireland remains the largest source of U.S. drug imports. In 2012, Ireland sent more than $21.7 billion worth of drugs to the U.S., accounting for nearly one-fourth of the country’s imports. That’s nearly two times the value of the drugs imported that year from China, India, Japan and Singapore combined. (See BioWorld Today, Jan. 31, 2014.)
Other European countries that outpace their Asian counterparts in terms of drugs manufactured for the U.S. market are Germany, with 2012 drug exports of about $10.7 billion; Switzerland, with nearly $8.5 billion; and the UK, with about $6.2 billion.
Together, Germany, Ireland, Switzerland and the UK accounted for more than half of the drugs the U.S. imported in 2012.
