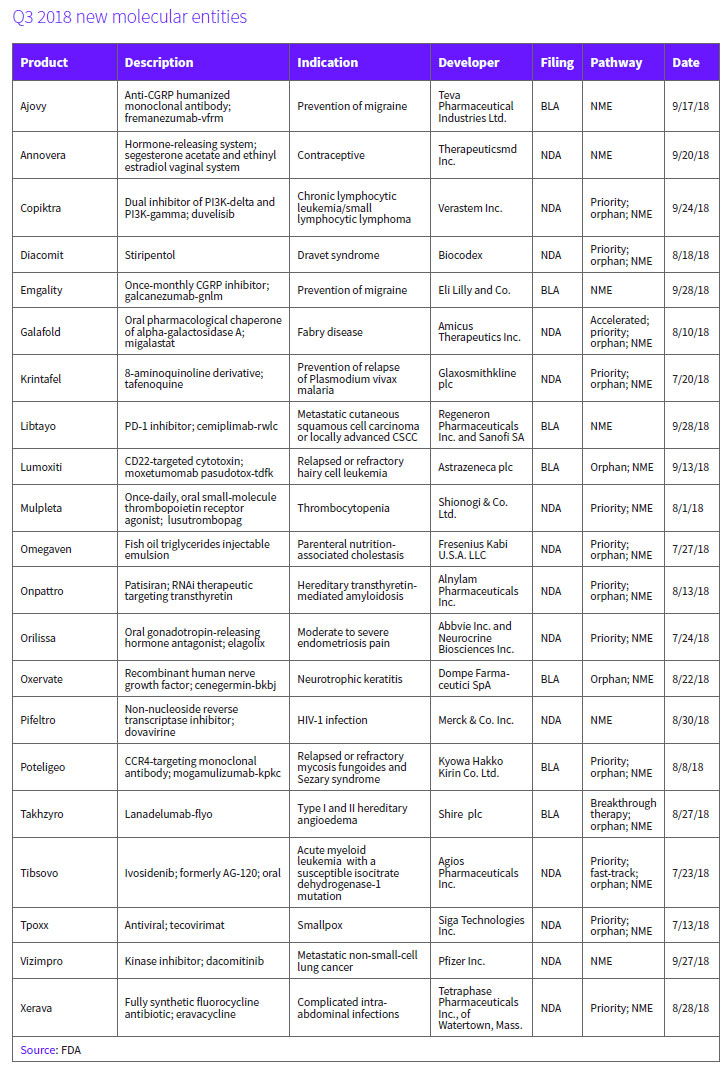
It was a productive third quarter for biopharmaceutical companies, which brought 21 new molecular entities (NMEs) across the FDA's goal line, the most approvals in any quarter since 2012. The approvals add to the 20 NMEs that have already received the agency's green light this year. The industry is now well-positioned to break its recent high watermark for new drug approvals established last year at 46, a total that currently ranks as the second highest annual total of all time, behind the 1996 total when 53 new medicines were approved. (See BioWorld Insight, Jan. 8, 2018.)
In fact, the record is poised to fall quickly – likely early in the fourth quarter – thanks to four approvals so far in October, including antisense oligonucleotide Tegsedi (inotersen) for polyneuropathy in adults with hereditary transthyretin-mediated amyloidosis, from Ionis Pharmaceuticals Inc. and affiliate Akcea Therapeutics Inc., as well as Revcovi (elapegademase-lvlr), a new recombinant enzyme replacement therapy for ultra-rare genetic disorder adenosine deaminase severe combined immune deficiency, from a U.S. subsidiary of Italy's privately held Leadiant Biosciences SpA. Also, in short order, Paratek Pharmaceuticals Inc. brought two new medicines to market with its sarecycline, branded Seysara, to treat inflammatory lesions of non-nodular moderate to severe acne vulgaris, and, omadacycline, branded Nuzyra, as a once-daily intravenous (I.V.) and oral antibiotic to treat adults with community-acquired bacterial pneumonia and those with acute skin and skin structure infections, becoming the first such product to gain full approval in both indications and formulations in nearly 20 years. (See BioWorld, Oct. 4, 2018.)
Innovation in overdrive
The total of 21 new medicines represent the highest number of approvals achieved in a third quarter period during the past six years. (See New molecular entities approved, below.)
Among the approvals were two targeting migraine. Eli Lilly and Co.'s migraine prevention therapy, Emgality (galcanezumab-gnlm), in fact, represented the third calcitonin gene-related peptide (CGRP) to receive the FDA green light following on a September approval for Teva Pharmaceutical Industries Ltd.'s Ajovy (fremanezumab-vfrm) and, back in May, for Amgen Inc.'s Aimovig (erenumab-aooe).
They enter a fiercely competitive market where global sales are estimated to grow at a compound annual growth rate of more than 10 percent, according to several analysts' reports.
A Cortellis consensus forecast, drawn from the predictions of five analysts, projects sales of $650 million annually for Emgality by 2022, nearly in line with predictions for Ajovy sales. Revenues from Amgen's Aimovig, by contrast, are expected to build to $1.26 billion over the same period.
A further competitor could enter the field next year with Bothell, Wash.-based Alder Biopharmaceuticals Inc. reporting in its second-quarter financials that it remains on track to submit a BLA in the first quarter of next year for its CGRP product, eptinezumab. Difei Yang, analyst with Mizuho Securities, wrote in a research note that she continues to see eptinezumab as "positively differentiated from competing products given 1) a unique I.V. route of administration allowing for quarterly dosing, 2) potential reimbursement under medical benefits, and 3) attractive responder rates with a higher proportion of patients being migraine-free after treatment administration vs. competitors."
Crowded market
The PD-1 market also has an increasing number of products reaching the market, the latest of which was Libtayo (cemiplimab-rwlc, previously REGN-2810) developed by Regeneron Pharmaceuticals Inc. and Sanofi SA. The anti-PD-1 sneaked under the third-quarter wire, a month ahead of its PDUFA date, as the first FDA-approved therapy to treat advanced cutaneous squamous cell carcinoma (CSCC) or locally advanced CSCC in individuals who are not candidates for curative surgery or radiation. (See BioWorld, Oct. 2, 2018.)
The fully human monoclonal antibody added to the market availability of the already FDA-approved anti-PD-1 agents, including Keytruda (pembrolizumab, Merck & Co. Inc.) and Opdivo (nivolumab, Bristol-Myers Squibb Co.).
Several other therapeutics targeting cancer were also approved. (See Q3 2018 new molecular entities, below.)
Ahead of an Oct. 5 PDUFA date, Needham, Mass-based Verastem Inc. won FDA clearance for Copiktra in adults with relapsed or refractory CLL/small lymphocytic lymphoma after at least two prior therapies. An oral inhibitor of phosphoinositide 3-kinase (PI3K), Copiktra is the first approved dual inhibitor of PI3K-delta and PI3K-gamma, enzymes that help malignant B cells grow. The drug won accelerated approval and hit the market with a list price of $11,800 per month, and is given twice daily in a 25-mg dose. (See BioWorld, Sept. 26, 2018.)
At the end of the quarter, Pfizer Inc. gained approval for the oral kinase inhibitor Vizimpro (dacomitinib) to treat individuals with metastatic non-small-cell lung cancer with EGFR exon 19 deletion or exon 21 L858R substitution mutations as detected by an FDA-approved companion diagnostic.
Another pharma, Cambridge, U.K.-based Astrazeneca plc and its Medimmune unit, gained approval for Lumoxiti (moxetumomab pasudotox-tdfk), the first new drug to be marketed for hairy cell leukemia in more than two decades. The CD22-targeted cytotoxin is indicated for adults with relapsed or refractory disease who have received at least two prior systemic therapies, including a purine nucleoside analogue. (See BioWorld, Sept. 14, 2018.)
Tibsovo (ivosidenib, formerly AG-120) received FDA approval in July to treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 mutation. The oral drug, developed by Agios Pharmaceuticals Inc., became the first approved therapy in the targeted indication and the first in the class that will be used with an approved companion diagnostic, the Abbott Realtime IDH1 test. (See BioWorld, July 23, 2018.)
Orphan designation
Among the NMEs approved, 12 had orphan drug designations, including Siga Technologies Inc.'s Tpoxx (tecovirimat), an oral antiviral against smallpox caused by variola virus in adult and pediatric patients, and Kyowa Hakko Kirin Co. Ltd.'s CCR4-targeting monoclonal antibody for use in two types of non-Hodgkin's lymphoma, including Sezary syndrome, for which it became the first approved therapy. Branded Poteligeo (mogamulizumab-kpkc), the drug also received a breakthrough therapy designation, one of the 12 NMEs that received this designation from the FDA in the quarter.