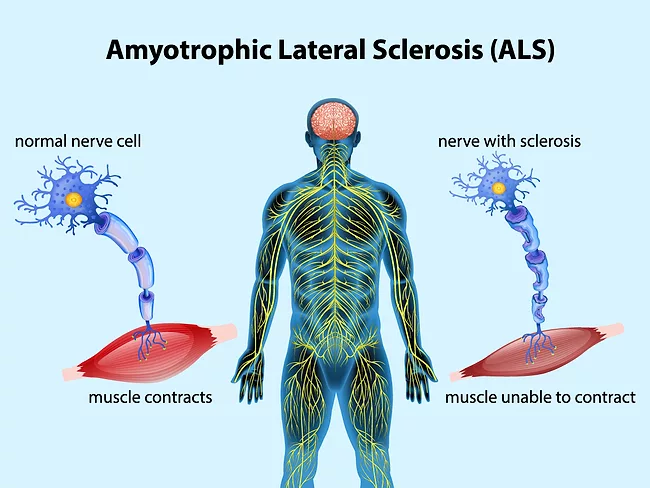
Articles Tagged with ''amyotrophic lateral sclerosis''
Neurology/Psychiatric
Cav2 calcium channel modifier GV-58 shows beneficial effects in experimental ALS
Read MoreNeurology/Psychiatric
Ractigen's therapeutic siRNA RAG-17 awarded US orphan drug designation for ALS
Read MoreNeurology/Psychiatric