BioWorld - Saturday, December 27, 2025
ARTICLES
EuroPCR 2025
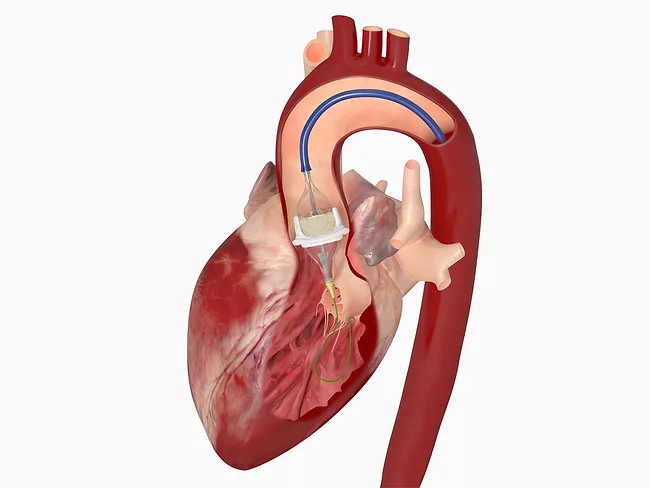
Edwards' data shows benefits of early intervention in aortic stenosis
May 23, 2025
EuroPCR 2025
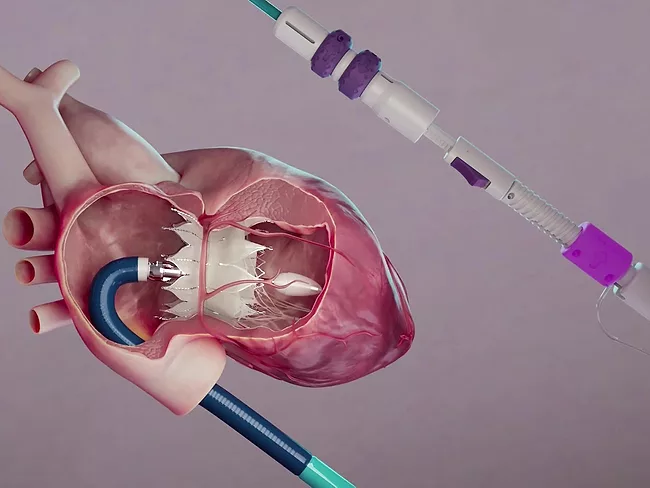
Tricares Topaz valve significantly reduces tricuspid regurgitation
May 22, 2025