CDU Contributing Writer
TORONTO – The 31st annual meeting of the Society of Interventional Radiology (SIR; Fairfax, Virginia) covered new developments in a wide range of areas, consistent with the expanding range of minimally invasive therapies provided by interventional radiologists and the diversity of the technologies employed in the field. Two areas highlighted at the conference that address major disease categories in the U.S. and worldwide – less invasive treatments for stroke and cancer – encompass a number of novel new technologies and represent expanding segments of the medical device market.
In some cases, such as the use of high frequency focused ultrasound (HIFU) for ablation of tumors as well as the use of stents and stent-grafts to treat vascular disease, technology developments are allowing other disciplines such as vascular surgeons and cardiologists to perform interventional radiology procedures, and in some cases those developments threaten the role of interventional radiology as a separate discipline.
As treatment modalities become increasingly noninvasive and diagnostic technologies become easier to use, a broader range of physicians are able to perform procedures that formerly were the domain of interventional radiology. At the same time, however, new applications have emerged, driving continued growth in the field. Considerable emphasis is evident on methods to improve the treatment of Deep Venous Thrombosis (DVT) and other venous conditions, and orthopedic applications also represent a growth area.
Some applications that were expected to become high-growth segments of the market have not expanded as predicted, such as carotid stenting, where growth has been hampered by reimbursement issues and technology limitations. Nevertheless, the overall rate of procedure growth in interventional radiology remains above 10% per year in the U.S., resulting in growing demand for products in the sector.
Stroke treatment challenge
The treatment of stroke, including interventional approaches for stroke prevention, represents a major area of opportunity in interventional radiology. More than 750,000 strokes occur annually in the U.S., and most of those who have a stroke do not receive effective treatment. There are about 4 million stroke survivors in the U.S., and 15% require full-time institutional care. About 30% have needs for assistance with daily living, and 60% suffer some loss of physical and/or mental function.
More reimbursement is becoming available for stroke management, however, because it has been demonstrated that existing treatments such as thrombolysis can improve outcomes if initiated on a timely basis. In addition, new technologies are continuing to emerge that may provide benefits to stroke patients by reducing the degree of disability resulting from a stroke, or that assist in stroke rehabilitation.
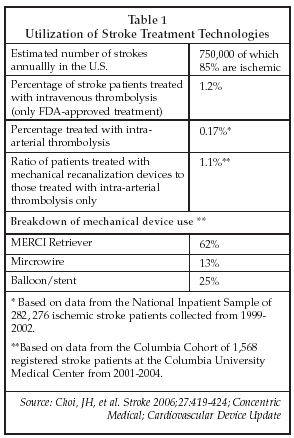
At present, as shown in Table 1, only 1.2% of patients who suffer an ischemic stroke are treated with FDA-approved intravenous thrombolytic therapy, and about one-10th of 1% are treated with catheter-directed intra-arterial thrombolysis. An equal number are treated with endovascular revascularization techniques employing mechanical devices, with somewhat less than two-thirds of those patients treated with the Merci clot removal catheter sold by Concentric Medical (Mountain View, California). The Merci catheter is the only mechanical device cleared by the FDA for removal of blood clots from the brain of patients suffering an ischemic stroke, and is particularly well suited for removal of large blood clots.
The reasons for the lack of treatment of stroke patients include failure of victims to seek treatment on a timely basis, as well as concerns about the safety of available therapies. Only 20% of patients present within three hours of stroke onset, the accepted window for initiation of thrombolytic therapy. In addition, many clinicians are reluctant to prescribe thrombolytic drugs due to the risk of hemorrhage. The latter barrier to treatment may be diminishing, however, because of the increasing efficacy of CT imaging to identify the source of an ischemic stroke and to identify patients who are contra-indicated for treatment.
Manufacturers of diagnostic imaging systems include Philips Medical Systems (Best, the Netherlands), GE Healthcare (Milwaukee), Siemens Medical Solutions (Erlangen, Germany) and Toshiba (Tokyo). Using CT systems from Philips, for example, radiologists can color-code brain tissues to indicate which areas are suitable for treatment and which are not treatable due to a pre-existing hemorrhage or other contra-indication. A typical protocol involves performing three CT exams including non-contrast CT, perfusion CT, and CT angiography in the span of less than 10 minutes. An MRI exam may also be performed as an adjunct to CT imaging since MRI is highly sensitive for detection of strokes, although compliance with the MR imaging procedure can be an issue for stroke patients.
Alternatives and issues
In addition, an increasing proportion of the population is now aware of stroke treatment alternatives, and patients’ families are increasingly likely to sue for malpractice if FDA-approved therapy is not provided. Some physicians consider existing mechanical devices such as the Merci catheter to be too stiff for use in the cerebral vessels, and cite occurrences of vessel rupture and device fracture as well as clot fragmentation as factors that make them reluctant to employ mechanical recanalization in stroke patients.
The cost of using the Merci device is also a factor, since some procedures may require as many as three catheters at a cost of around $2,000 each.
As discussed by JC Wojak, MD, of Stanford University Medical Center (Stanford, Connecticut), similar issues exist with newer devices now under evaluation for stroke therapy such as the Micro Infusion Catheter, an ultrasound-assisted drug delivery device from Ekos (Bothell, Washington), although the Ekos catheter has shown increased clot lysis and improvement in patient outcome in the IMS II trial.
ImaRx Therapeutics (Tucson, Arizona) also is developing an ultrasound-based system for stroke therapy, the SonoLysis system, which uses nanobubbles and ultrasound to break up clots in ischemic stroke, as well as the SonoLysis PROLYSE, which combines recombinant prourokinase with nanobubbles and ultrasound for combined mechanical and biochemical clot lysis.
Another device, the NeuroFlo catheter from CoAxia (Maple Grove, Minnesota), which received humanitarian device approval about a year ago, is available for the treatment of cerebral ischemia due to vasospasm, and uses a series of balloons to increase blood flow to the brain. However, because of the complexity of existing devices, and continued issues with catheter stiffness and fractures for recanalization devices, utilization is likely to be limited.
Device/drug strategies
Other applications for mechanical recanalization devices, including applications that combine devices with thrombolytic drugs to enhance clot dissolution, are treatment of deep venous thrombosis (DVT) and clearing of thrombosed dialysis access grafts. DVT therapy represents an opportunity for market expansion since the condition is under-diagnosed and under-treated, according to Suresh Vedantham, MD, of the Mallinckrodt Institute of Radiology (St. Louis), who cited data showing that less than 5% of eligible DVT patients undergo catheter-directed thrombolysis.
According to Victor Tapson, MD, of Duke University Medical Center (Durham, North Carolina), the failure to diagnose and treat DVT is one of the most common causes of medical liability lawsuits. About 15 million people in the U.S. have a history of DVT, and between 400,000 and 600,000 new cases are diagnosed each year.
Numerous devices exist for use in performing thrombus removal, including the AngioJet from Possis Medical (Minneapolis); the Helix, Xsizer and Amplatz thrombectomy catheters from ev3 (Plymouth, Minnesota); the Oasis system from Boston Scientific (Natick, Massachusetts), the ProLumen catheter from Datascope (Mahwah, New Jersey); the Resolution therapeutic wire from OmniSonics Medical (Wilmington, Massachusetts); the Trellis-8 from Bacchus Vascular (Santa Clara, California); and the PTD from Arrow International (Reading, Pennsylvania). However, none are approved specifically for treatment of DVT, according to Mahmood Razavi, MD, of the Mayo Clinic (Rochester, Minnesota).
One factor that limits the use of combination device/drug therapy for DVT, including catheter-directed thrombolysis, is cost, since hospitalization is required, whereas anti-coagulant therapy using approved drugs such as Lovenox, a low molecular weight heparin marketed by Sanofi Aventis (Paris), can be performed on an outpatient basis.
Combination device/drug therapy can be highly effective, however, particularly for well-organized thrombus where the use of targeted, localized delivery of thrombolytic drugs combined with mechanical or ultrasound disruption of a clot can allow lower systemic drug exposure and reduce the risk of hemorrhage.
At present, there is essentially no data from randomized trials that demonstrates the safety and efficacy of device/drug therapy compared to drug therapy alone, but a number of trials are in early stages that will compare the two modalities.
Mechanical techniques
Registry data, discussed by Vedantham at the SIR conference, indicates a high (88%) success rate for clot lysis using catheter-based techniques. An important advantage of using mechanical techniques to treat DVT is that more extensive clearance of clot can be achieved than with drug therapy alone, and residual thrombus is reduced, minimizing the risk of post-thrombotic syndrome and vein damage.
Companies developing device-based technologies for DVT therapy include Bacchus Vascular, Ekos, Possis Medical, and OmniSonics. Bacchus received FDA clearance about a year ago for the Trellis-8, a device that employs dual balloons and a rotating wire to create a chamber in the vessel in which the clot can be isolated and macerated. Drugs are also infused into the chamber once the balloons are inflated to aid in lysis of thrombus. Enrollment in the STRIDE (does early Safe Thrombus Removal In Dvt have an Effect on patient outcome) single-arm registry was scheduled to begin in May 2006 to assess patient outcome in DVT using endovascular therapy with the Trellis-8 plus anti-coagulant therapy.
Ekos believes its $2,000 ultrasound-based device will have a particularly important advantage in treating DVT since the ultrasound energy penetrates venous valves to loosen clot behind the valve, which is not possible using other clot removal methods. OmniSonics is also employing ultrasound for clot dissolution in its Resolution system, which received FDA clearance in December 2005 for use in clearing thrombus from dialysis access grafts. The $600 device consists of a guide catheter and a .009” titanium wire driven with low-power ultrasonic energy by the Resolution generator to produce oscillations in the wire that break up thrombus but have no impact on red blood cells or other elastic cells.
The device also does not damage the vessel wall. About 100 procedures have been performed with the device so far, and good success rates have been achieved in clearing organized thrombus, including some grafts that have been thrombosed for up to three months. OmniSonics is now evaluating applications of its technology for DVT therapy.
New intervention at the periphery
Treatment of peripheral vascular disease is another growing application in interventional radiology. One area that is the focus of considerable development effort at present is device-based therapies for occlusive disease in the lower extremities. Lower extremity disease represents a major health issue, since occlusive disease of the femoro-popliteal arteries comprises over 50% of all cases of peripheral arterial disease, and results in 300,000 amputations annually in the U.S.
Surgical bypass is used to treat many patients with lower extremity arterial occlusions and stenosis, but this is traumatic and results in patency rates of only about 80% at one year. Balloon angioplasty is less invasive than bypass surgery, but patency rates have been poor. New approaches are employing stents and stent-grafts for the treatment of small-vessel occlusive disease in the superficial femoral and femoro-popliteal arteries.
The Viabahn stent-graft, manufactured by W.L. Gore (Flagstaff, Arizona), is one device that has been evaluated. Studies cited at the SIR conference show three-year patency rates of 79% for the Viabahn in the SFA, equivalent to rates for surgical bypass using vein grafts, and significantly better than rates if synthetic bypass grafts are employed.
In principle, the ePTFE covering on the Viabahn helps prevent tissue in-growth, improving long-term patency compared to that achievable with bare metal stents, which is the other modality being developed for treatment of lower extremity occlusive disease.
The Viabahn has the mechanical flexibility required to withstand the flexure stresses characteristic of the SFA, and coverage of collateral vessels by the stent-graft material has not proven to be a major issue, according to users.
Nevertheless, as discussed by Mark Mewissen, MD, of St. Lukes Vascular Center (Milwaukee) at a symposium sponsored by W.L. Gore held during the SIR conference, bare metal stents still have a role in the SFA. Mewissen, who has used the SMART stent from Johnson & Johnson’s Cordis (Miami Lakes, Florida) unit extensively in the SFA, as well as the SelfX stent from Abbott Vascular Devices (Redwood City, California) and the Luminexx stent from Bard Peripheral Vascular (Tempe, Arizona), has found that bare metal stents can be placed in vessels that cannot be accessed using the Viabahn.
In addition, Mewissen believes blockage of collateral vessels as occurs with covered stent-grafts in the lower extremities can prove detrimental, noting that some cases of critical limb ischemia have been documented for patients treated with the Viabahn, whereas such events are very rare in his experience with bare metal stents.
Other physicians have evaluated the Hemobahn, another covered stent-graft from W.L. Gore, in long lesions in the SFA with good results. Thomas Zander, MD, of the Hospiten Rambla (Santa Cruz de Tenerife, Spain) reported 78.4% primary patency at four years using the Hemobahn in long lesions in the SFA, compared to only 70% patency for surgical bypass at four years. Zander is now conducting a randomized trial comparing the Hemobahn to surgery.
Stent grafting
Stent grafts have also been used with success in the treatment of failed dialysis access grafts. A. Marcovici, MD, of New York Presbyterian/Columbia University Medical Center (New York), reported on the use of an ePTFE-encapsulated Nitinol stent-graft from Bard Peripheral Vascular in dialysis access grafts at the SIR conference. Results from a randomized study involving 97 patients found a statistically significant improvement in patency for stent-grafts compared to angioplasty. In addition, in stent-grafts that failed, the pattern of re-occlusion was different from that observed with angioplasty in that most of the stenoses were short and occurred in cases in which two overlapping grafts were used, indicating that longer devices may provide even better results.
At present, Bard markets the Fluency stent-graft worldwide with an approved indication for treatment of tracheobronchial stenosis. Another new development in stent-graft technology was described by Cook, which exhibited a new fenestrated version of the Zenith stent-graft for use in treatment of abdominal aortic aneurysm (AAA) that allows stenting of aneurysms that extend to the renal arteries. The device allows additional short covered stents to be placed in the renal arteries that overlap with the main body of the AAA stent-graft to provide complete coverage.
An aneurysm extending to the renal arteries presents a challenge when using a conventional AAA stent-graft since it would block the entrance to the renal arteries if placed as needed to seal the aneurysm. The new device is already available in Europe, Australia, and Canada for compassionate use, but is not yet available in the U.S.
Stenting the carotids
The status of carotid artery stenting was another topic highlighted at the SIR conference. The use of carotid artery stents to prevent stroke has been viewed as the second largest potential market for stents (after coronary stents) by suppliers. However, market growth has lagged since devices were approved by the FDA for carotid use.
According to physicians who discussed carotid stenting at the SIR conference, current reimbursement policies have created a significant barrier to widespread use of carotid stenting. At present, reimbursement is limited to patients who have a 70% or greater stenosis, restricting the target patient population to a small percentage of the 140,000 to 175,000 patients who undergo treatment for carotid stenosis annually.
However, as discussed by Mark Wholey, MD, of the University of Texas Health Science Center (San Antonio) at a symposium sponsored by Boston Scientific, deficiencies in existing carotid stent technology may also be partly to blame for the low utilization of carotid stenting. Noting that a 2% rate of procedure-related stroke, increasing to 16% in patients age 80 and over, continues to be observed in studies of carotid stenting conducted after market introduction.
Wholey said he believes that improved devices are needed in order to justify widespread use. Existing carotid stent delivery systems are not sufficiently flexible and maneuverable to safely cross the aortic arch in some patients, and self-expanding stents are difficult to place with the precision needed to achieve consistently good results. In addition, embolic protection filters used with existing FDA-cleared carotid stents still allow particles to pass downstream and into the brain.
Wholey advocates the use of embolic protection devices that incorporate flow reversal to eliminate procedure-related emboli, and he also is experimenting with new stents employing closed-cell designs and nanopore technology as well as balloon-expandable devices.
A positive finding for carotid stenting was, however, also presented at the SIR conference. Rodney Raabe, MD, of Sacred Heart Medical Center (Spokane, Washington) described a study that was intended to demonstrate the need for embolic protection devices to prevent cognitive defects in carotid stenting.
The study not only showed the need for protection devices, but also found that carotid stenting improves cognitive ability in patients with severe stenosis, both for patients who were symptomatic for cerebral ischemia and well as those who were asymptomatic prior to undergoing the stent procedure. The improvement in cognitive ability was attributed to improved blood flow to the brain. The results could broaden the indications for carotid stenting.
