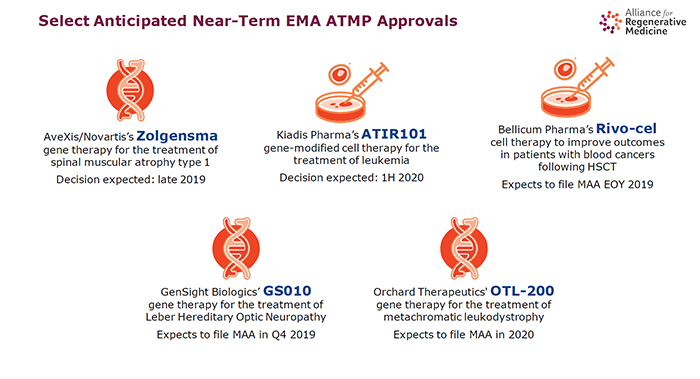
LONDON – With five expensive advanced therapies recently approved in Europe and a further five expected to get approval in the near term, it is vital the hurdles that delay and preclude access – and which have led to the commercial failure of earlier products – are removed.
"The pipeline is extremely full and a significant number of these products are coming to market in the next five years. We know that without removing the barriers, people won't get access," said Janet Lambert, CEO of the advocacy group Alliance for Regenerative Medicine (ARM).
"For us, this feels like a really important moment in the history of ATMPs [advanced therapy medicinal products]," Lambert said, launching a report, titled "Getting Ready," which surveys the landscape in Europe and makes recommendations to improve adoption.
Europe has been a scientific and regulatory leader, being first to create a framework for approvals and to give the green light to an autologous cell therapy, Tigenix NV's Chondrocelect; to the first gene therapy to be approved in a regulated market, Uniqure NV's Glybera (alipogene tiparvovec); and to the first stem cell product, Holoclar.
All three were home grown and each provided incremental improvement; also, none were/are profitable. Only one patient received the marketed form of Glybera, which cost $1.2 million for a one-off treatment, between approval in July 2012 and Uniqure's decision not to renew the license in 2017. (See BioWorld, April 21, 2017.)
Chondrocelect, for treating knee cartilage defects, was withdrawn in 2016. Holoclar, an autologous product for treating burns to the eye, is still registered.
"Exciting products have come to market but have been withdrawn because they were not a commercial success," Lambert said.
ARM's 330 members funded the report, investigating the current position on market access in cell and gene therapies and tissue engineered products in France, Germany, Italy, Spain, Sweden and the U.K. In the light of that, the report makes a series of recommendations on how to create a receptive environment.
One of the key findings is that Europe's national health technology assessment (HTA) bodies do not have the tools to put a value on products, which, while bearing very high price tags, typically require a single administration that has profound and long-lasting effects.
Much work needs to be done in fine-tuning HTA methodologies, said Lambert. "There is a real need to better adapt HTA to ATMPs," she said. That process is likely to take some time.
In the meantime, ARM is calling for wider application of conditional or managed access schemes, in cases where the – usually limited – evidence is showing a potential strong benefit, but without long enough follow-up to establish the size and duration of effect.
That approach is used in England, for example, where reimbursement for the CAR T therapies Kymriah (tisagenlecleucel) and Yescarta (axicabtagene ciloleucel) is conditional on gathering in-market data, allowing for future price negotiations.
"We understand that payers don't have all the information they have [with traditional products], when deciding on access," Lambert said.
There needs to be a pan-European effort to create a system for real-world evidence collection, to follow patients over time, she said. "Existing infrastructure tends to be very distributed and run by scientific societies or patient advocacy groups; there is not a federated way of tracking [patients]."
It would also be helpful if there was a channel for companies to have an early dialogue with payers and HTA agencies in advance of marketing approval, to ensure the right data are collected in clinical development and that there is awareness of what is coming down the pipeline. Lambert said currently there is not enough bandwidth in the system to allow for those conversations to take place.
At the same time, ATMPs are usually administered in centers of excellence, of which there may not be one in every country. There is an urgent requirement for measures to coordinate and fund access to cross-border ATMP treatments at a European level.
Finally, there need to be new payment models, such as pay for performance, annuity schemes spreading payments over several years, or special funds set up to pay for ATMPs and shield the rest of the health care system from their cost impacts.
There is "some real willingness" from HTAs and payers to grant access, but complex alternative payment systems will take some time to put in place, said Lambert. "Working with other stakeholders, we will begin the process of engagement with national players, to try to address each recommendation," she said.
"Folks don't quite appreciate how many of these products are in the pipeline and how soon they might be faced with [them]."